The impact of the direction of the chemical bond of colliding molecules on their chemical reactions has been observed in collisions of non-polar molecules for the first time. This observation and its theoretical underpinnings potentially offer a new way to control chemical reactions beyond the traditional chemical-reaction parameters of temperature, pressure, and the addition of catalysts.
Researchers have observed steric effects, the interactions of molecules depending on their spatial orientation (not just between their electrons involved in bonding), in a chemical reaction involving non-polar molecules for the first time. The breakthrough opens the door to an entirely new way to control the products of chemical reactions.
Chair Professor Xueming Yang from the College of Science at the Southern University of Science and Technology (SUSTech), in collaboration with the theory team of Prof. Donghui Zhang at the Dalian Institute of Chemical Physics (Chinese Academy of Sciences), recently published their research about accurate control of stereodynamical effects in chemical reactions
Their research article, entitled “Stereodynamical control of the H + HD → H2 + D reaction through HD reagent alignment,” was published in Science. The reviewer believes that this work is an important milestone in the field of reaction dynamics.
One of the central goals of chemistry is to develop new methods of controlling chemical reactions. For the most part, control of chemical reactions involves understanding the interactions between the electrons of different atoms. These “electronic” effects govern many of the properties and behavior of chemicals and the changes they undergo during reactions.
But the relative spatial arrangement of atoms within molecules also impacts their properties, behavior, and the changes they can undergo. The study of how these spatial arrangements affect chemical reactions, so-called “steric effects”, and how to manipulate such effects make up the subdiscipline known as stereochemistry, sometimes called “3D chemistry” for its focus on the orientations of atoms and molecules in three-dimensional space.
These effects are the result of repulsive forces between overlapping electron clouds. The complement of opposite and like charges produce a particular 3D shape in a molecule, including the angles between atoms and the axis of the chemical bond (bond axis).
The researchers specialized in attempting to control the direction of the bond axis of molecules involved in chemical reactions—a way to manipulate chemical reactions beyond traditional methods, such as adding suitable catalysts and changing the temperature or pressure of a reaction mixture.
In turn, the mutual orientation of reactants colliding with each other also has a major effect on the outcome of the chemical reaction. Thus, by controlling that orientation, it should be possible to promote or restrict the yield of the products of the reactions into specific final states or scattering angles.
Experiments attempting to use the stereodynamical effects to achieve control of chemical reaction processes and results have been performed for several years.
Molecular hydrogen (H2), a non-polar molecule, is a good candidate for such orientation-control scattering experiments, not least due to its simplicity of each atom having just one proton and one electron (and thus enjoying considerable theoretical tractability). However, until recently, it has been difficult to prepare sufficient concentrations of H2 in specific quantum states for scattering experiments.
With the development of a high pulsed energy and single-longitudinal mode optical parametric oscillator/amplifier, large concentrations of H2 in specific quantum states have become available using the scheme of stimulated Raman pumping. The quantity is now sufficient to study collision dynamics and to align H2 molecules in a particular direction for steric dynamics experiments.
With the advent of this new technique, the researchers wanted to see whether the predicted significant steric effects could be observed in the simplest chemical reactions involving H2 molecules.
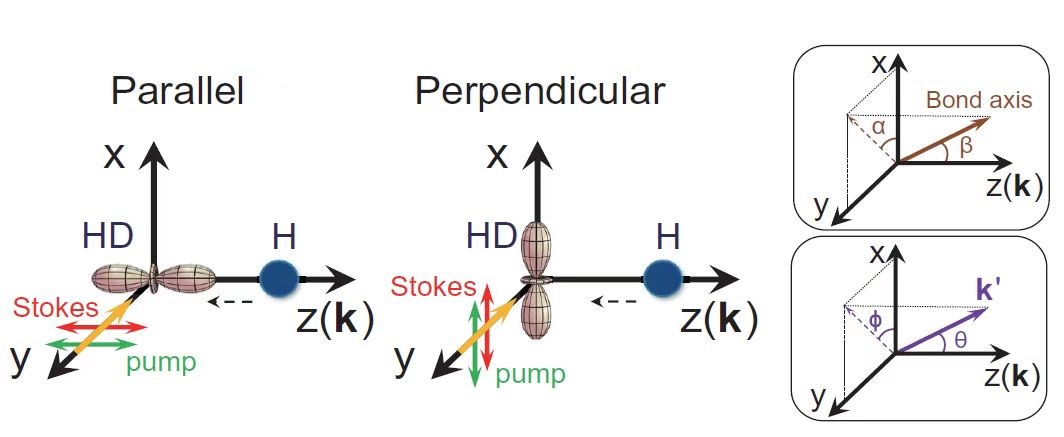
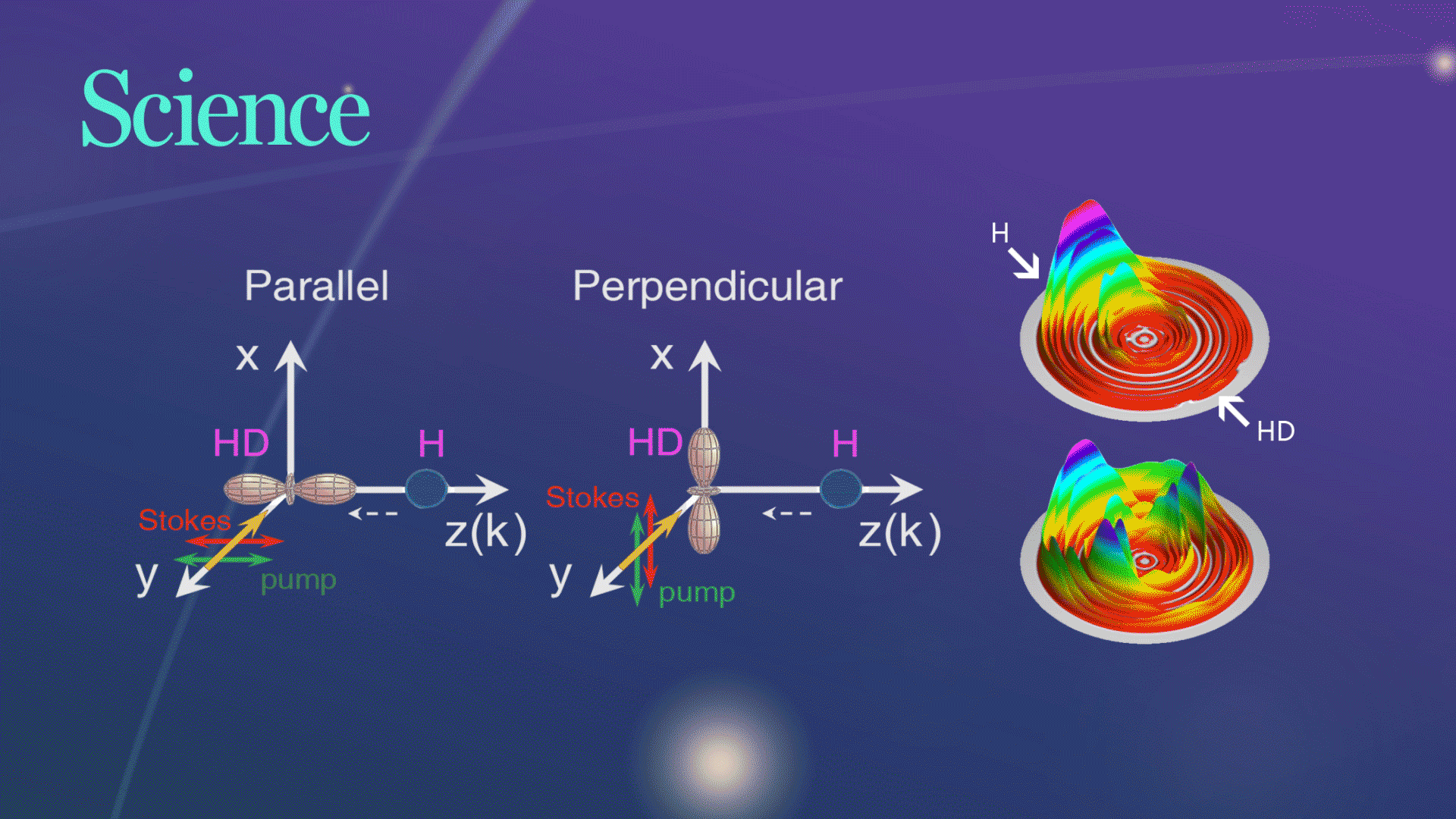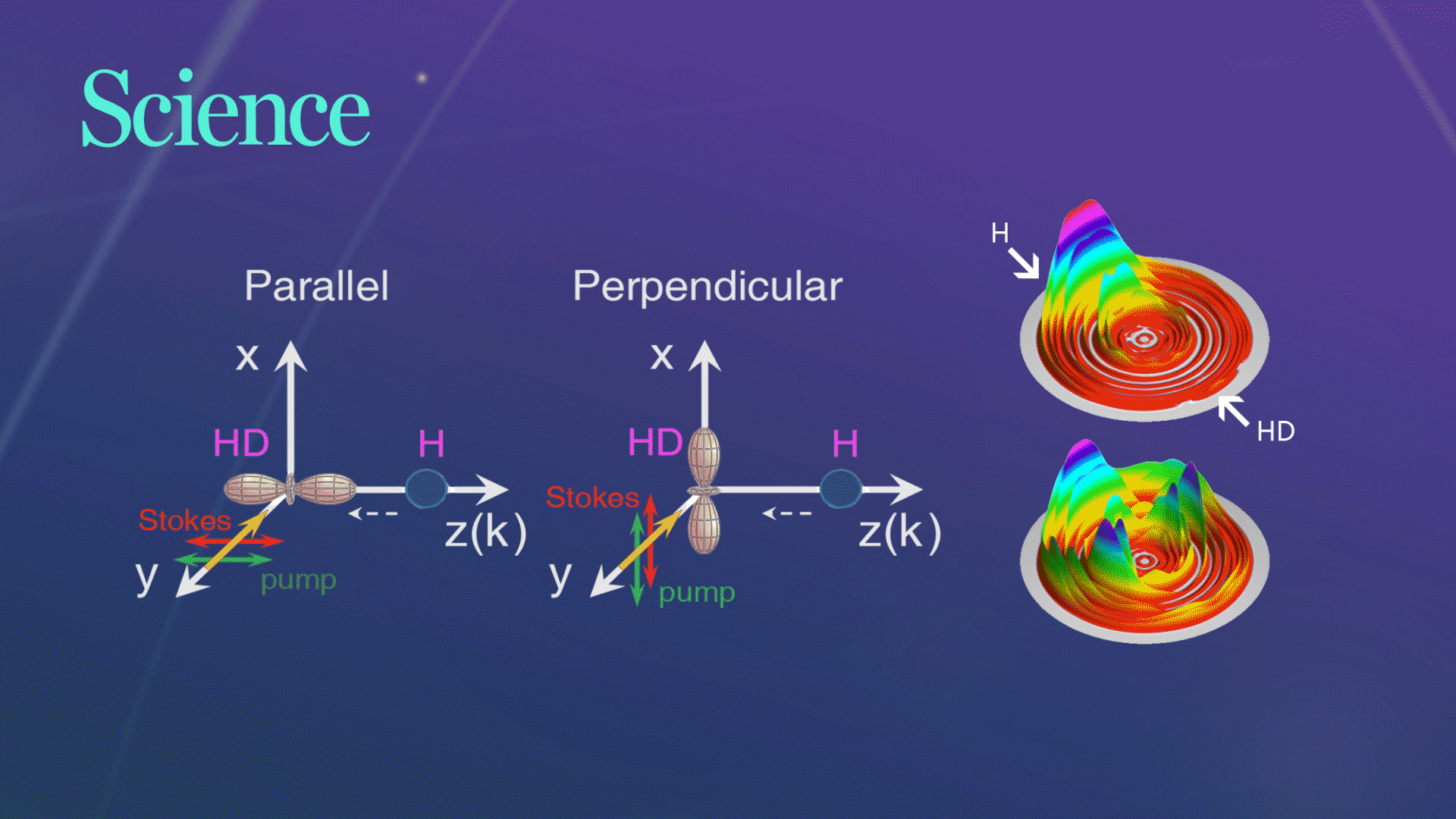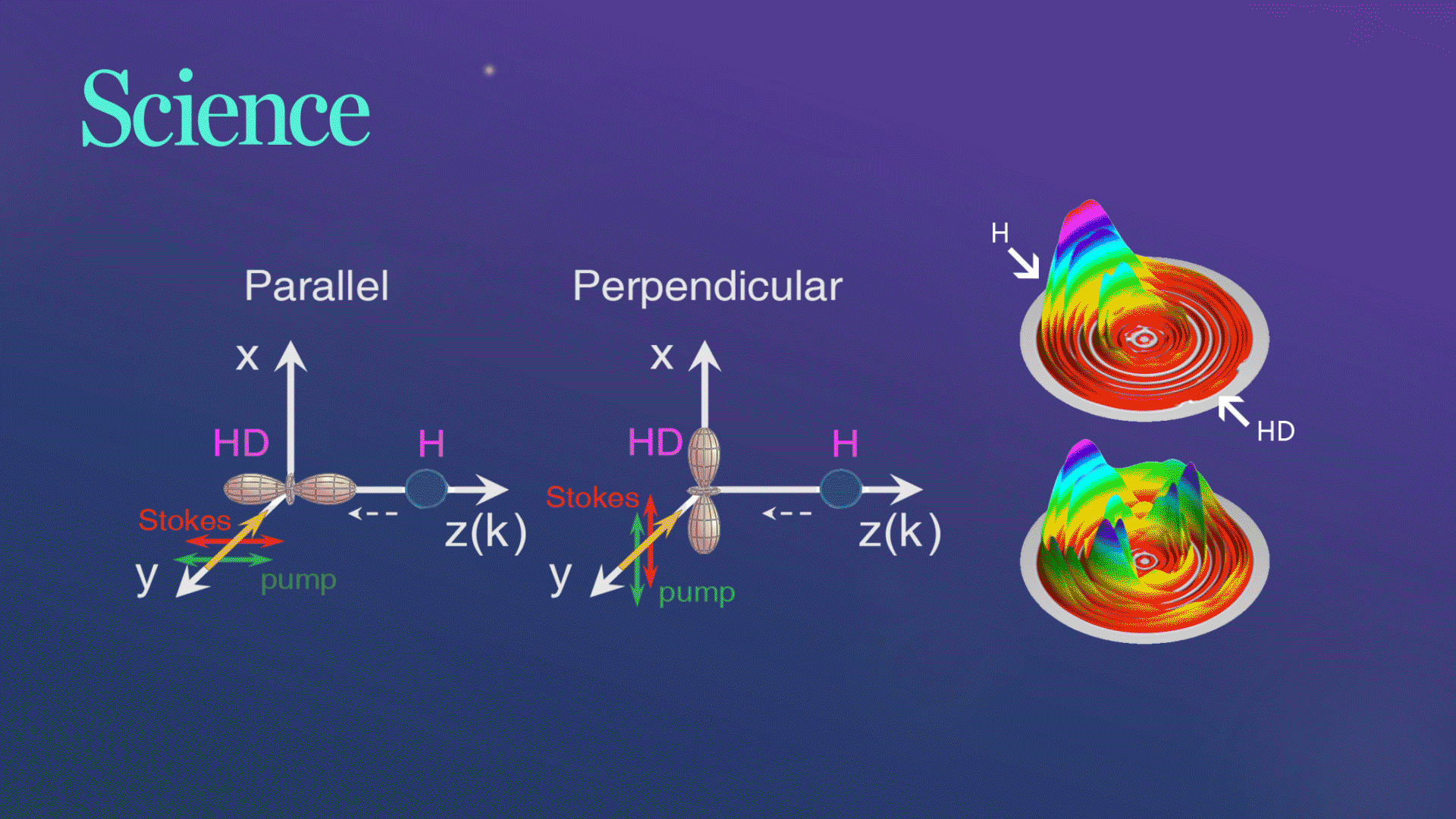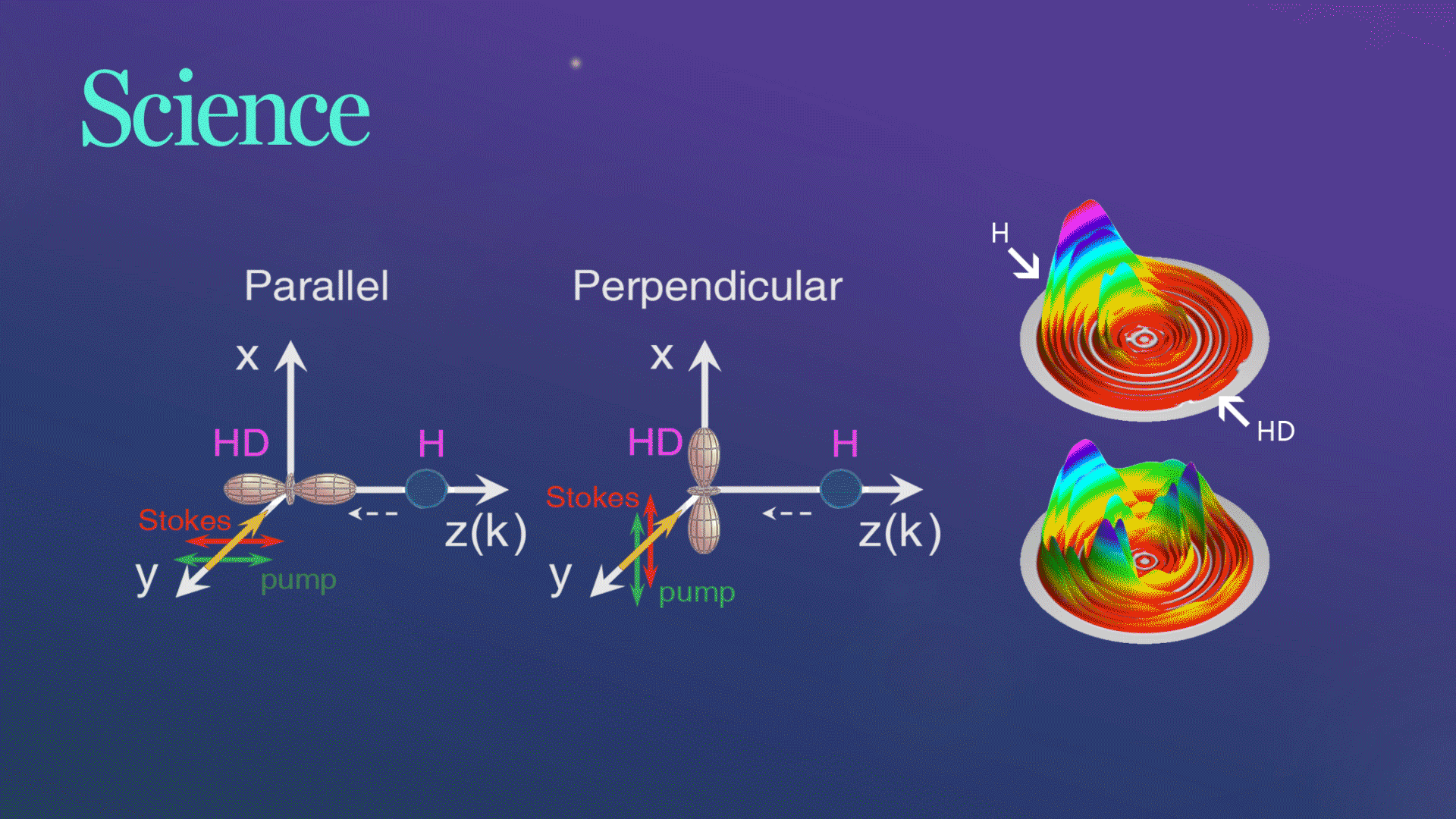
Figure 1. The schematic of two collision geometries prepared by SRP
For their experiment, the researchers picked a very simple chemical reaction, the transformation of hydrogen (H) and hydrogen deuteride (HD)—a hydrogen atom bonded to an atom of deuterium, an isotope of hydrogen with an extra neutron, into molecular hydrogen (H2) and deuterium (D). They collided the chemical reactants at three different collision energies and at two different collision geometries, one where the chemical bond was aligned parallel to the relative velocity of the collision partners and one where the bond was aligned in a perpendicular fashion.

Figure 2. Three-dimensional scattering H2productcontour plots on the scattering plane
The research team successfully observed the hypothesized substantial steric effects while using quantum dynamics calculations to analyze their observations in a straightforward manner. They found that when two waves produce a larger wave in the perpendicular configuration, it plays an important role in the observed steric effects. The chemical reaction changed drastically depending on the direction of the HD bond axis.
The observations and the underlying theoretical understanding opens the door to a new way to control chemical reactions.
Prof. Xueming Yang, Prof. Dong H. Zhang, Dr. Zhaojun Zhang, and Prof. Chunlei Xiao are the corresponding authors of this paper.
This work was supported by the Innovation Program for Quantum Science and Technology, National Natural Science Foundation of China (NSFC), and Scientific Instrument Developing Project of the Chinese Academy of Sciences.
Paper link: http://www.science.org/doi/10.1126/science.ade7471
To read all stories about SUSTech science, subscribe to the monthly SUSTech Newsletter.
Proofread ByAdrian Cremin, Yingying XIA
Photo By