Metal-catalyzed carbon-carbon cross-coupling reactions have evolved into one of the major technologies for C−C bond-forming events. Over the past decades, reductive cross-coupling between two carbon electrophiles has become an attractive alternative for C−C bond-forming due to the sole use of bench-stable and cost-effective carbo-electrophiles as coupling precursors.
In conventional reductive cross-coupling reactions, C−C bond-forming process occurs exactly at the position of reactive functional groups preinstalled on coupling partners. Moreover, methods amenable for remote C−C bond cross-coupling occur at unfunctionalized positions of alkyl chains, allowing for building saturated structures difficult to access otherwise. The cross-electrophile coupling between two migratory alkyl halides imposes additional challenges on chemo-, and site-selectivity of alkyl-alkyl bond formation.

Associate Professor Wei Shu’s research team of the Department of Chemistry at the Southern University of Science and Technology (SUSTech) has recently made significant progress toward metal-catalyzed regiodivergent cross-electrophile alkyl-alkyl couplings of alkyl halides.
Their research, entitled “Ligand-Controlled Nickel-Catalyzed Regiodivergent Cross-Electrophile Alkyl-Alkyl Couplings of Alkyl Halides,” was published online in Angewandte Chemie International Edition, one of the prime chemistry journals in the world.
Diverse site selectivities have been realized using alkyl bromides by judicious choice of ligand and conditions to tune the migration of alkyl nickel species and alkyl-alkyl coupling. The reaction features exclusive control of the migration over non-migration between two migratory alkyl bromides as well as reactivity differentiation between several similar alkyl-Ni species to afford β-, γ-, δ-alkylated amides from identical starting materials (Figure 1).
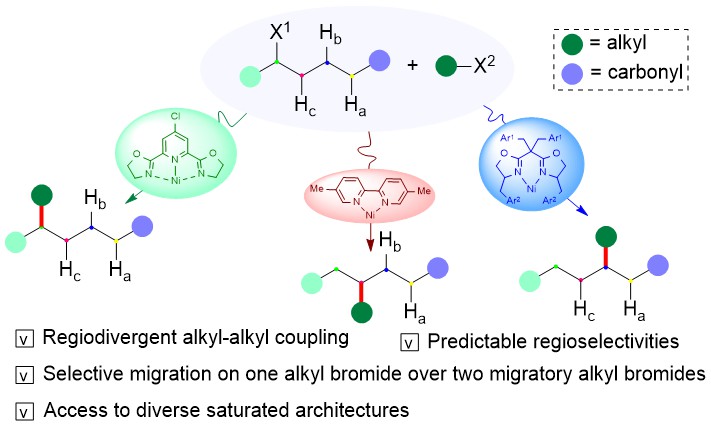
Figure 1. Ligand-Controlled Regiodivergent Cross-Electrophile Alkyl-Alkyl Couplings
The reaction has good compatibility with various functional groups such as ether, ester, ketone, aldehyde, chlorine, trifluoromethyl, etc.. The catalytic method also has good applicability for N-alkyl amides and N, N-disubstituted amides. In addition, secondary halogenated compounds are also suitable for this reaction condition (Figure 2). The work is highlighted in Synfats (Synfats, 2023, 19, 0372).
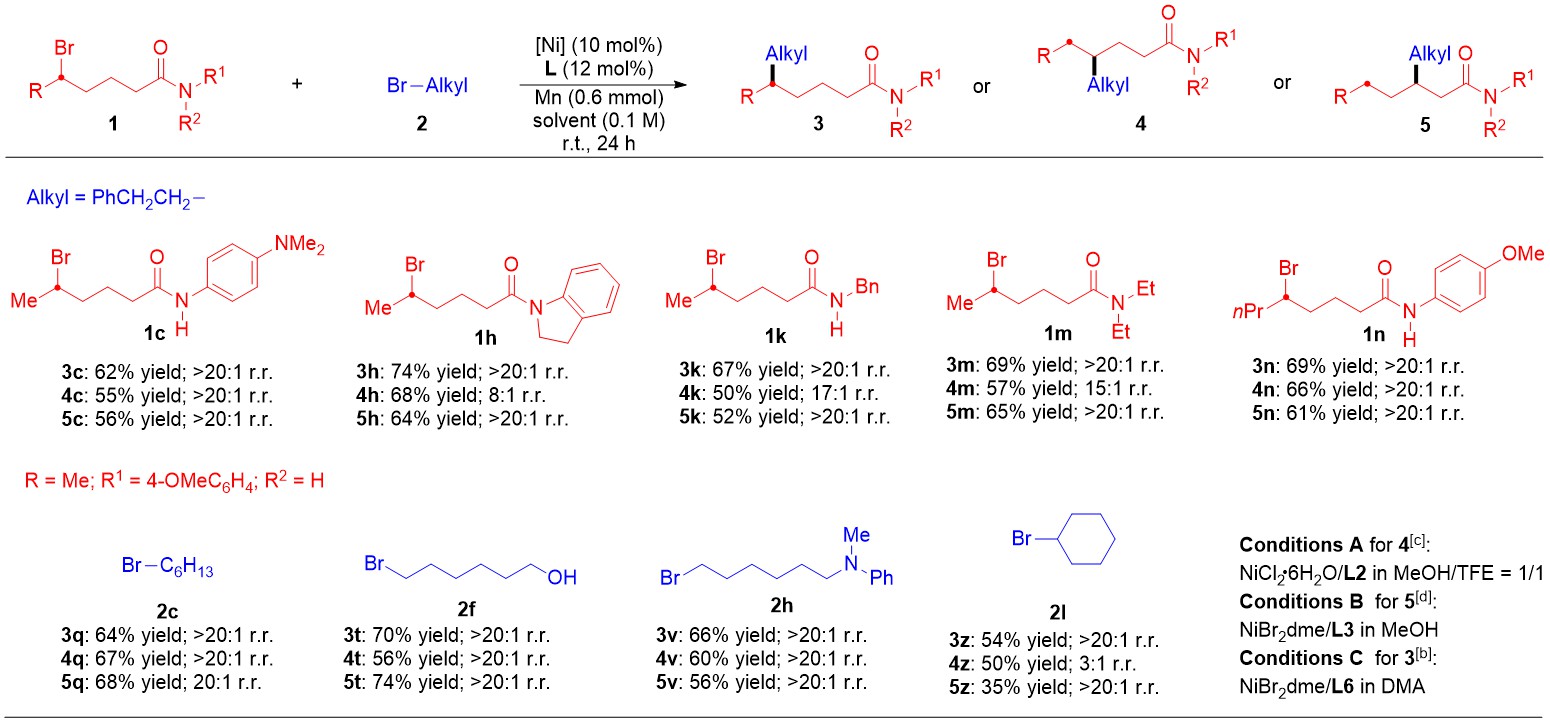
Figure 2. Selected Examples for Regiodivergent Cross-Electrophile Alkyl-Alkyl Couplings
The above-mentioned research was conducted at SUSTech. Wen-Tao Zhao is the first author of this paper. Assoc. Prof. Wei Shu is the corresponding author.
This work was supported by the National Natural Science Foundation of China (NSFC), National Natural Science Foundation of Guangdong, Guangdong Provincial Key Laboratory of Catalysis, Shenzhen Peacock Program, and the Shenzhen Science, Technology and Innovation Commission. The researchers also acknowledge the assistance of the SUSTech Core Research Facilities.
Paper link: https://doi.org/10.1002/anie.202215779
To read all stories about SUSTech science, subscribe to the monthly SUSTech Newsletter.
Proofread ByAdrian Cremin, Yingying XIA
Photo By