Ribosomes are the core machinery for protein synthesis within cells, and their assembly involves the intricate regulation of numerous assembly factors. Although YjgA had been previously reported to participate in the late-stage assembly of the 50S subunit, its precise mechanism of action remained unclear.

Professor Fuxing Zeng’s research group from the Department of Systems Biology, School of Life Sciences at the Southern University of Science and Technology (SUSTech) has recently published a study that employs cryo-electron microscopy and biochemical techniques, elucidates the crucial functions of the small protein YjgA during the late-stage assembly of the 50S ribosomal subunit, providing novel insights into the complex process of ribosome biogenesis.
Their work, entitled “YjgA plays dual roles in enhancing PTC maturation”, has been published in Nucleic Acids Research, a journal covering leading-edge research into physical, chemical, biochemical, and biological aspects of nucleic acids and proteins involved in nucleic acid metabolism and/or interactions.
Through knockout studies of YjgA or its N-loop expression in Escherichia coli, the researchers observed the accumulation of pre50S particles, which predominantly displayed structural abnormalities in the peptidyl transferase center (PTC) and the H68/69 region. Cryo-electron microscopy data analysis revealed eight distinct conformations of pre50S-ΔyjgA and six of pre50S-ΔNloop, highlighting the critical role of YjgA-Nloop in integrating the uL16 protein and stabilizing the H89 region within the PTC.
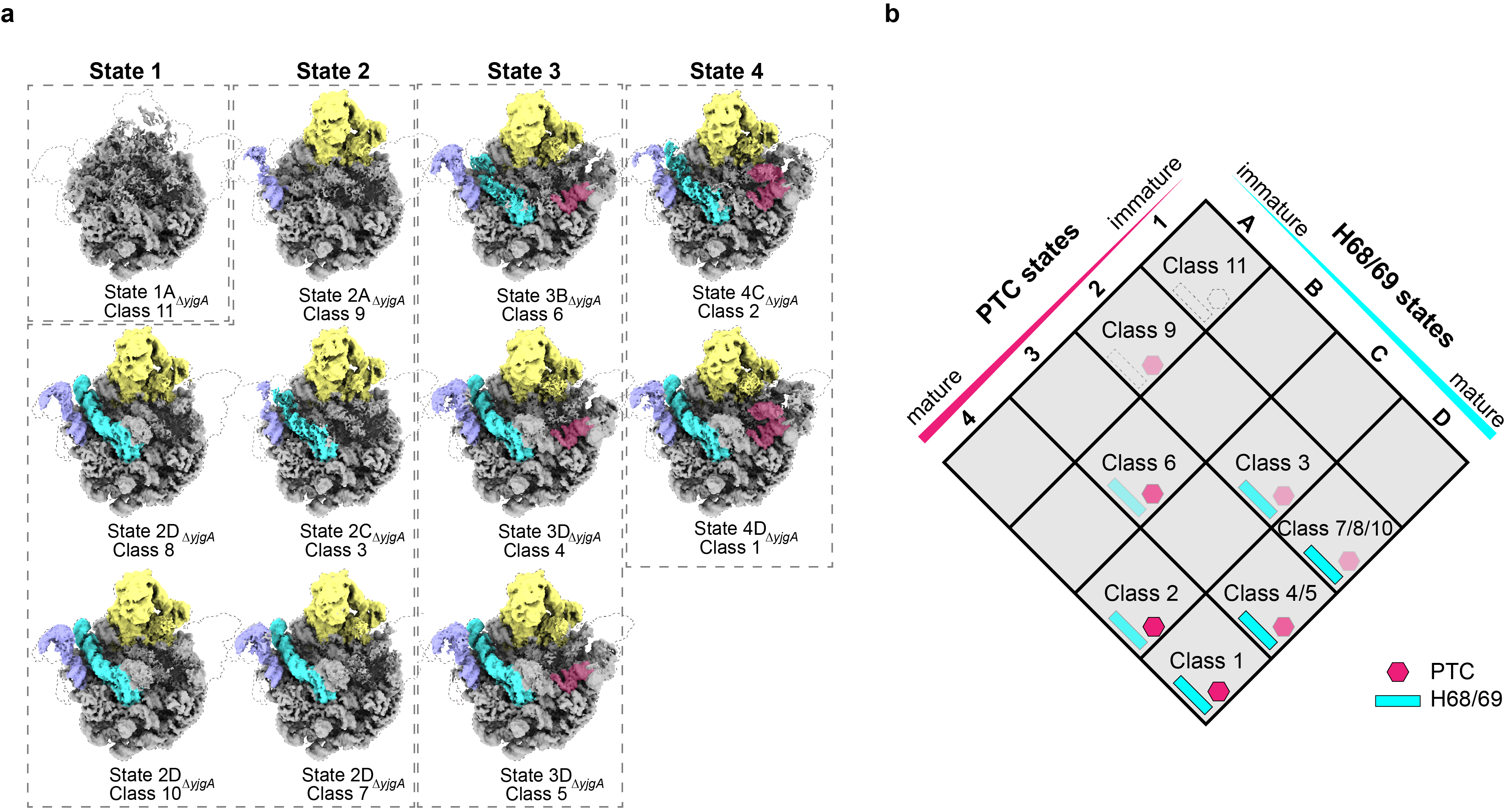
Figure 1. Different conformations of pre50S-ΔyjgA, revealing that the absence of yjgA primarily affects the formation of the PTC and H68/69
Further experiments, including pull-down assays with uL16, confirmed this mechanistic role of YjgA. Additionally, the CTLH-like domain at the C-terminus of YjgA was found to inhibit the docking of H68 by binding to the base of the L1 stalk, providing the necessary space and flexibility for PTC folding, thereby facilitating PTC maturation.
This study illustrates how YjgA, through the coordinated action of its distinct domains, ensures the correct maturation of the PTC, which is critical for understanding how ribosomes are assembled into highly efficient functional complexes from their constituent parts.
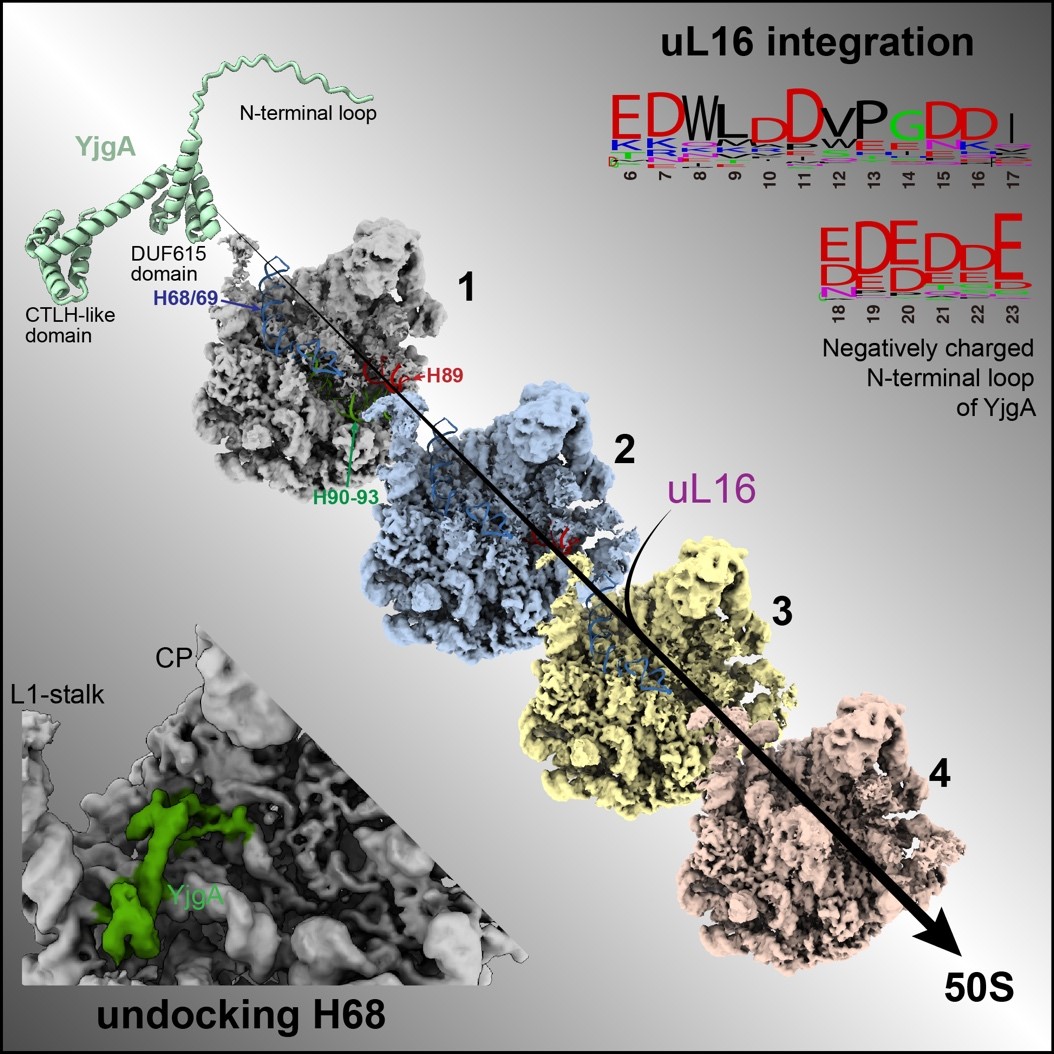
Figure 2. Dual roles of YjgA in promoting PTC maturation by preventing H68 docking and recruiting uL16
Doctoral student Mengtan Du at SUSTech is the first author of the paper. Professor Fuxing Zeng is the corresponding author. Other contributors to this work included undergraduate student Chenke Deng, doctoral student Ting Yu, and master’s student Qixin Zhou.
This research was supported by the National Natural Science Foundation of China (NSFC) and the Shenzhen Science and Technology Program. The collection and processing of cryo-electron microscopy data were provided by the Cryo-Electron Microscopy Center at SUSTech.
Paper link: https://academic.oup.com/nar/advance-article/doi/10.1093/nar/gkae469/7688991
To read all stories about SUSTech science, subscribe to the monthly SUSTech Newsletter.
Proofread ByAdrian Cremin, Yingying XIA
Photo BySchool of Life Sciences