Phytoalexins are chemical defense compounds evolved by plants to resist biotic stresses, such as pathogens and pests. Legumes, especially soybeans, uniquely synthesize isoflavonoid phytoalexins. Glyceollins, a class of soybean-specific isoflavones, exhibit strong antimicrobial activity and hold potential for drug development in cancer and antimicrobial therapies. However, glyceollins are present in trace amounts in soybeans, making chemical synthesis and plant extraction impractical. While synthetic biology offers a green manufacturing route, the complexity of their biosynthetic pathways—particularly the unknown final enzymatic step catalyzing the cyclization of the D to E ring by glyceollin synthases (GSs)—has hindered large-scale production and functional studies.
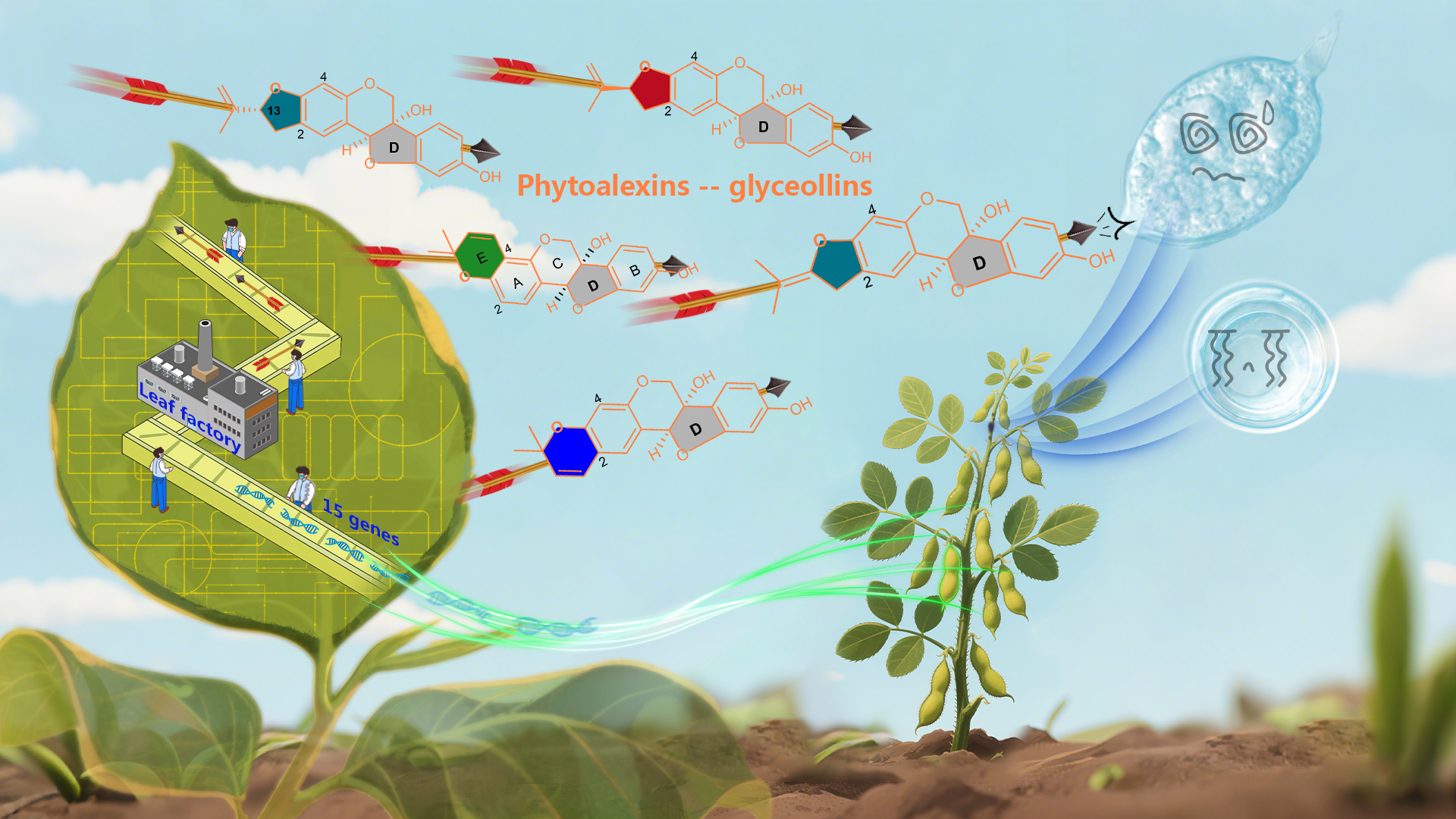
A groundbreaking study led by Associate Professor Ancheng Huang from the Department of Biology at the Southern University of Science and Technology (SUSTech) has pioneered a synthetic biology platform using engineered Nicotiana benthamiana plants to achieve high-yield production of isoflavones. The platform enabled the accumulation of key isoflavones, genistein and daidzein, at impressive yields of 11.8 g/kg and 7.0 g/kg (dry weight), respectively.
Using multi-omics technologies, the team deciphered the complete biosynthetic pathways of six antimicrobial phytoalexins in soybean, including glyceollins—among them the novel glyceollin VII. They achieved efficient de novo biosynthesis of glyceollins and other bioactive isoflavones in N.nthamiana. Notably, glyceollin I and II reached yields of 2.6 g/kg and 5.9 g/kg in dry leaves, respectively. This study advances plant synthetic biology chassis and promotes the bio-manufacturing of active isoflavones in plant systems.
Their paper, titled “Glyceollin biosynthesis in a plant chassis engineered for isoflavone production”, has been published in Nature Chemical Biology.
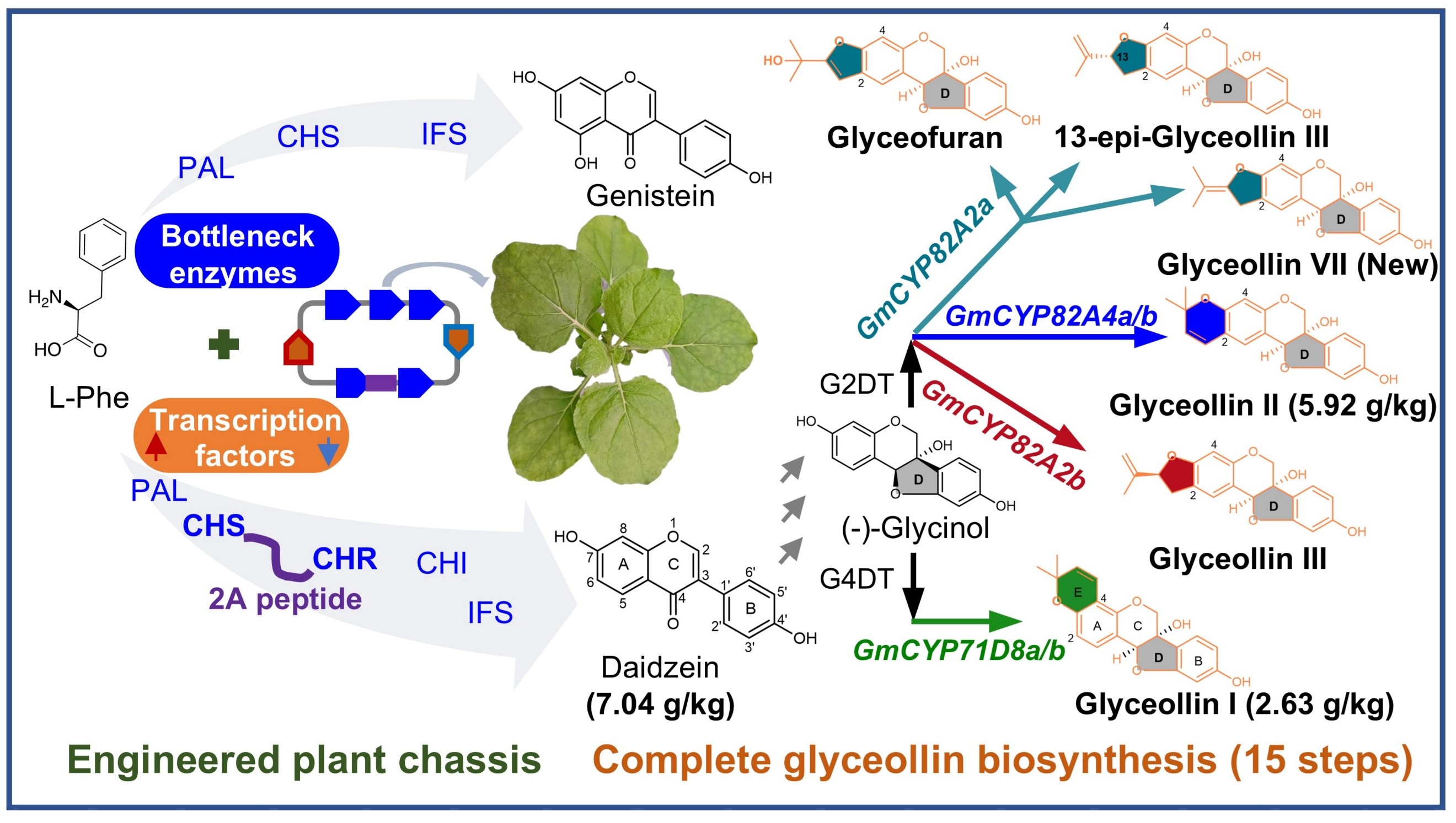
Figure 1. Developing a high-yield isoflavone plant chassis for synthesizing different glyceollins
The researchers reconstructed the upstream biosynthetic pathway of daidzein—a precursor of glyceollins—in N.benthamiana and employed a combination of strategies to enhance metabolic flux, including. They screened high-efficiency enzymes from a diverse range of plants, co-expressing key genes, and regulated metabolic flux via transcription factors. These efforts boosted isoflavone yields by 3- to 5-fold compared to simple overexpression of pathway genes, providing ample precursors for downstream glyceollin biosynthesis.
Using the high-yield N.benthamiana platform, the team identified six cytochrome P450 monooxygenases (GmGS1a/1b, GmGS2a/2b, GmGS3, and GmGS3e/7) responsible for forming the glyceollin E-ring. Functional validation confirmed their roles in synthesizing glyceollin I, II, III, VII, 13-epi-glyceollin III, and glyceofuran. CRISPR/Cas9-mediated knockout of these enzymes in soybean hairy roots revealed their necessity for glyceollin production during pathogen infection, establishing their biological roles for the first time.
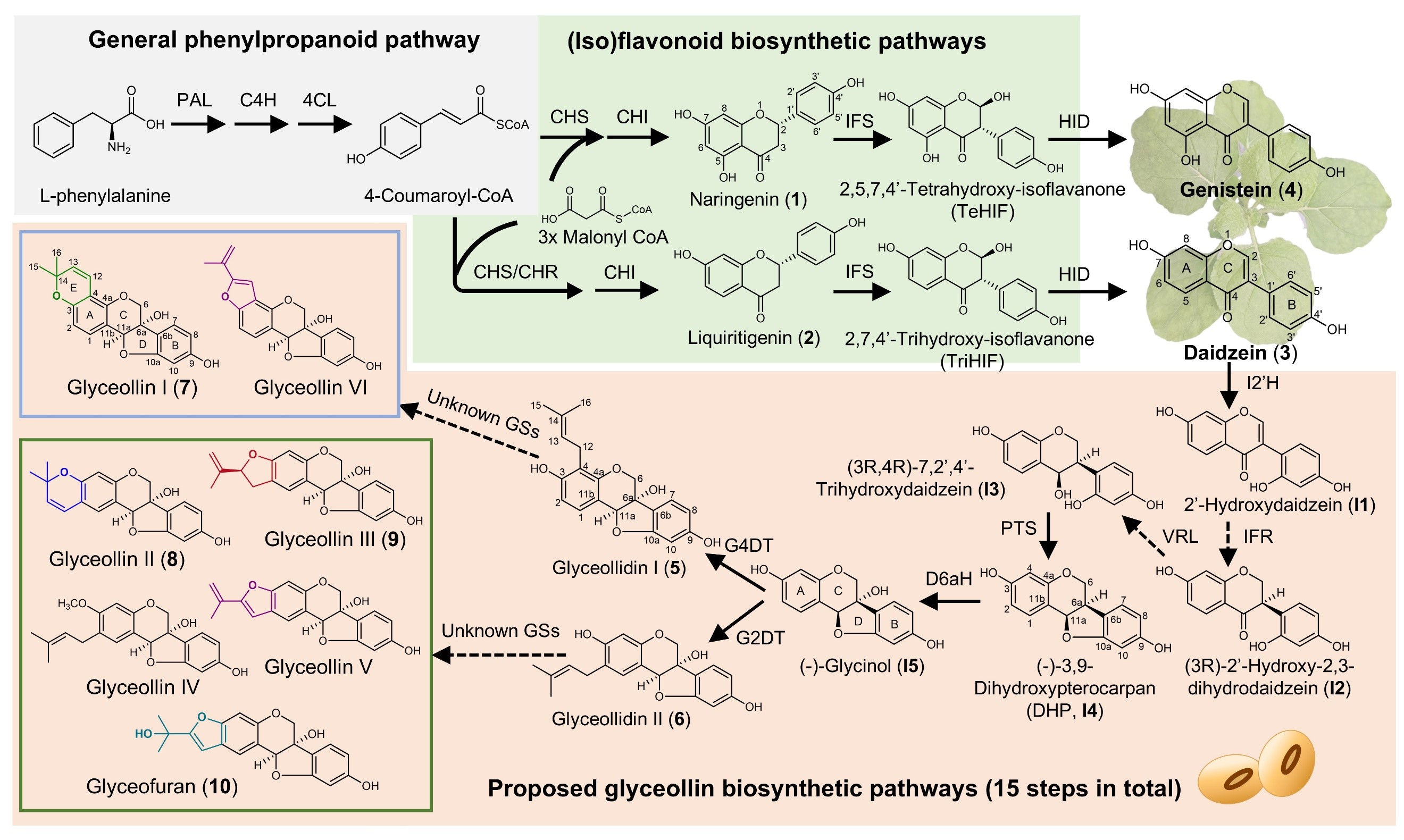
Figure 2. Biosynthesis pathway of glyceollins
The engineered N.benthamiana chassis enables green de novo synthesis of diverse bioactive isoflavones, including glyceollins (up to 5.9 g/kg in dry leaves) and medicarpin (0.7 g/kg). Antimicrobial assays demonstrated that 13-epi-glyceollin III and medicarpin strongly inhibit Phytophthora sojae and Phytophthora nicotianae, while glyceollin III showed weaker activity. These findings highlight the structure-activity relationship—particularly the critical role of C13 stereochemistry—and provide potential compounds for novel biopesticides.
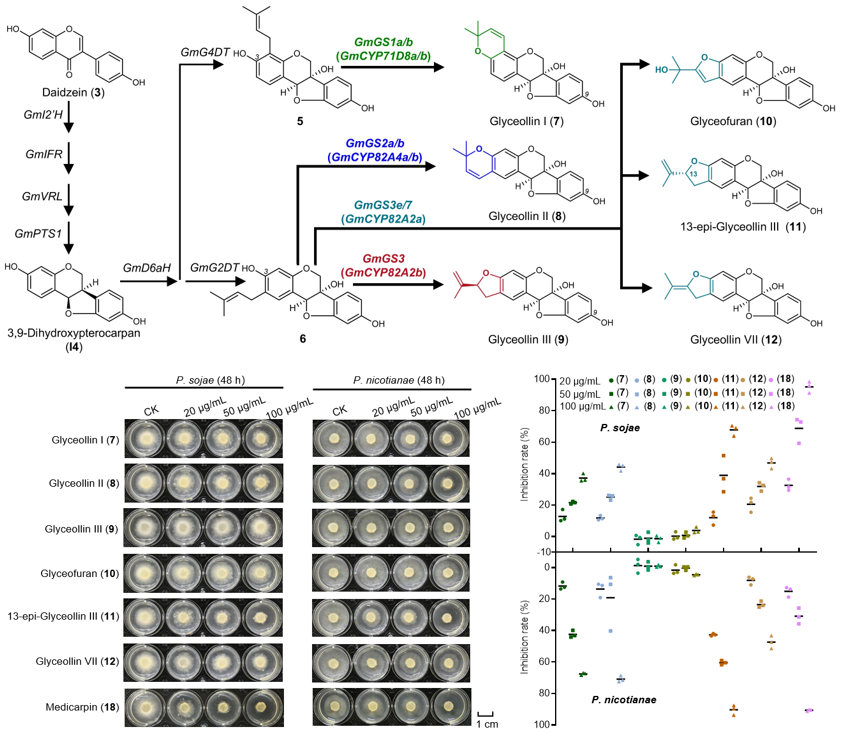
Figure 3. Identification of glyceollin synthases and antimicrobial assays of glyceollins and other isoflavones
This study decodes the complete glyceollin biosynthetic pathway in soybean and achieves heterologous biosynthesis in a plant chassis. It offers theoretical insights into plant defense mechanisms, genetic tools for breeding high-resistance soybean varieties, and sustainable solutions for agriculture. The developed chassis and technology also expand the sources of bioactive isoflavones for pharmaceutical and health-related applications.
Master’s graduate Jiali Xie and master’s student Jiayu Tian from SUSTech are the co-first authors of the paper. Associate Professor Ancheng Huang is the corresponding author, with SUSTech serving as the corresponding institution. Other contributors to this work include postdoctoral researchers Salman Khan, Yuqiong Hao, and Feilong Chen; Ph.D. candidate Jingwei Yu; Research Assistant Professor Qian Zhou; Professor Feng Zhang from Nanjing Agricultural University; and Associate Professor Guoyuan Zhu from the Macau University of Science and Technology.
Paper link: https://www.nature.com/articles/s41589-025-01914-3
To read all stories about SUSTech science, subscribe to the monthly SUSTech Newsletter.
Proofread ByAdrian Cremin, Yifei REN
Photo ByDepartment of Biology