The global spread of the monkeypox (mpox) outbreak has posed a severe threat to public health security, prompting the World Health Organization (WHO) to declare it a Public Health Emergency of International Concern (PHEIC) on two occasions. The A35 protein expressed on the enveloped virions (EVs) of the mpox virus is crucial for the intercellular transmission of the virus within the host, making it a potentially effective target for antiviral drugs.
Previous studies have shown that antibodies targeting the A35 protein of the mpox virus, or the homologous A33 protein of the vaccinia virus, can protect mice against poxvirus infection to varying degrees. With the continuous advancement of research on anti-mpox virus monoclonal antibodies, there remains a lack of relevant data regarding the protective efficacy of these antibodies in non-human primate models, and the molecular interaction mechanism by which antibodies recognize antigens also needs further clarification. Researchers at the Southern University of Science and Technology (SUSTech) have been working to address these gaps, driving progress toward improved understanding and potential therapeutic strategies against the mpox virus infection.

A team led by Chair Professor Zheng Zhang and Distinguished Researcher Bin Ju from the Shenzhen National Clinical Research Center for Infectious Disease at the Second Affiliated Hospital of SUSTech (Shenzhen Third People’s Hospital), in collaboration with Associate Professor Renhong Yan from the School of Medicine at SUSTech, together with colleagues from the Institute of Laboratory Animal Sciences (ILAS) of the Chinese Academy of Medical Sciences (CAMS) and the National Institute for Viral Disease Control and Prevention (NIVDC) of the Chinese Center for Disease Control and Prevention, has achieved progress in the development of anti-monkeypox virus monoclonal antibodies.
Their findings have been published in the journal Cell under the title “Structurally conserved human anti-A35 antibodies protect mice and macaques from mpox virus infection.”
The researchers identified two human monoclonal antibodies, mAb 975 and mAb 981, capable of cross-recognizing both the A33 protein of vaccinia virus and the A35 protein of the mpox virus. In the CAST/EiJ mouse infection model, the use of mAb 975 or mAb 981 alone exerted a significant protective effect against mpox virus infection, including reducing the viral load in mouse plasma and various tissues, as well as alleviating pathological damage to the mouse spleen. In the rhesus macaque infection model, mAb 975 and mAb 981 also demonstrated protective effects, such as decreasing the viral load in plasma, throat swabs, anal swabs, and various tissues, and most notably inhibiting the occurrence of skin lesions and pox formation in rhesus macaques.
High-resolution structures of the mAb 975-A35 and mAb 981-A35 complexes revealed the molecular mechanism by which the two antibodies recognize the mpox virus. By comparing these structures with the previously reported structure of a mouse-derived antibody complex (A27D7-A33), the team further summarized the conserved characteristics of monoclonal antibodies from different species in binding to the mpox A35 dimer or vaccinia A33 dimer. They found that antibodies can form stable electrostatic interactions between positively charged amino acids in the heavy-chain CDR3 region and negatively charged amino acids in the central part of the antigen dimer. Antibodies can also insert into the groove-like structure in the middle of the antigen dimer. Additionally, hydrophobic amino acids in multiple CDR regions of the antibody can cooperate with the hydrophobic platform of the antigen dimer and with the hydrophobic regions within the groove. Together, these interactions stabilize the antibody-antigen complex.
This study confirms that the two human anti-mpox virus monoclonal antibodies can protect mice and rhesus macaques against mpox virus infection to a certain extent, providing promising candidates for the development of antibody-based drugs. The binding model between the antibodies and the mpox A35 dimer clarifies the molecular interaction mechanism and also offers important references for the design of next-generation vaccines.
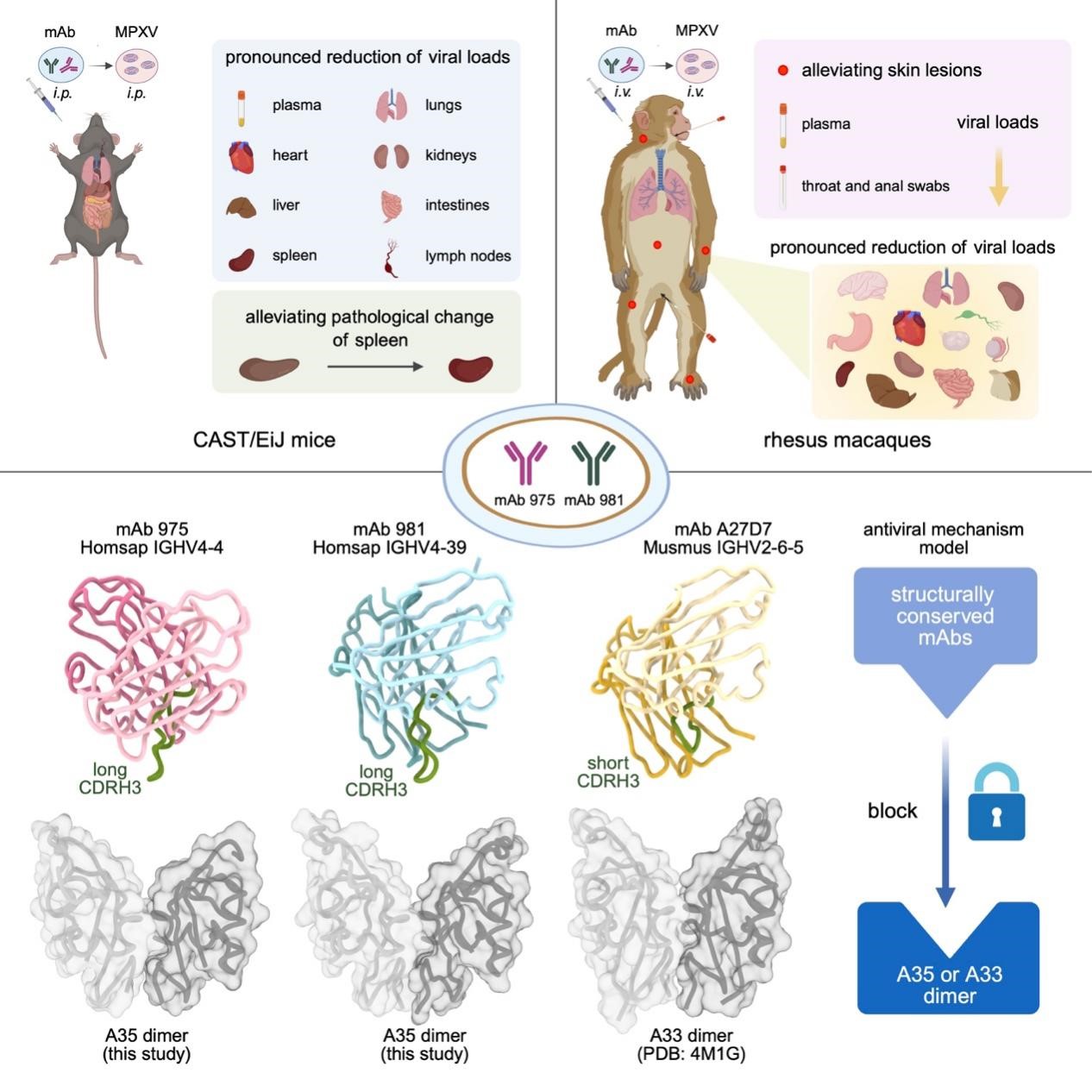
Figure 1. Structurally conserved human anti-A35 antibodies protect mice and macaques from mpox virus infection
Distinguished Researcher Bin Ju and Researcher Congcong Liu from the Shenzhen National Clinical Research Center for Infectious Disease at the Second Affiliated Hospital of SUSTech (Shenzhen Third People’s Hospital), Jingjing Zhang from ILAS, CAMS, Ph.D. graduate student Yaning Li from Tsinghua University, Ph.D. student Haonan Yang from SUSTech, Associate Researcher Bing Zhou from Shenzhen Third People’s Hospital, and Researcher Baoying Huang from NIVDC, Chinese Center for Disease Control and Prevention, are the co-first authors of this paper.
Chair Professor Zheng Zhang, Distinguished Researcher Bin Ju, Professor Jing Xue from ILAS, CAMS, Associate Professor Renhong Yan, and Professor Wenjie Tan from NIVDC, Chinese Center for Disease Control and Prevention, are the co-corresponding authors.
Paper link: https://www.cell.com/cell/abstract/S0092-8674(25)00919-5
To read all stories about SUSTech science, subscribe to the monthly SUSTech Newsletter.
Proofread ByAdrian Cremin, Yifei REN
Photo ByYan QIU