Resolving the fundamental conflict between high energy density and safety remains a formidable challenge in the research of lithium batteries. To date, a series of high thermal resistance additives, such as ethylene sulfate (DTD) and methylene methanedisulfonate (MMDS), have been extensively applied in industrial production with the purpose of enhancing SEI thermal stability and raising the threshold temperature for thermal runaway (TR). However, the efficacy of this approach is confined to the process defined as TR-1, and proves ineffective when the cell is brought to total combustion in the extreme abuse scenarios.
Flame-retardant additives, including free radical quenching organics (e.g., organophosphorus), have proven highly effective in neutralizing a considerable number of free radicals, which are generated from the decomposition of solvents and the failure of the anode and cathode electrodes and are critical in propagating combustion. In most cases, these organic phosphorus additives also bring compromised electrochemical performance. All-solid-state electrolytes (ASSEs), known for their high dimensional and thermal stability as well as prominent mechanical strength, are an ideal safety solution. Nonetheless, large-scale applications of ASSE have been hindered by many issues ranging from fundamental sciences to engineering challenges, and balancing high security with excellent electrochemical performance continues to be difficult in existing electrolyte systems.
In recent years, a series of novel thermoresponsive electrolytes have been developed. Those unique electrolytes can physically or chemically transform into insulating states at specified temperatures, preventing catastrophic thermal hazards. However, these design strategies for TR mitigation frequently entail the use of an entirely novel electrolyte system, which reduces their universality and feasibility.
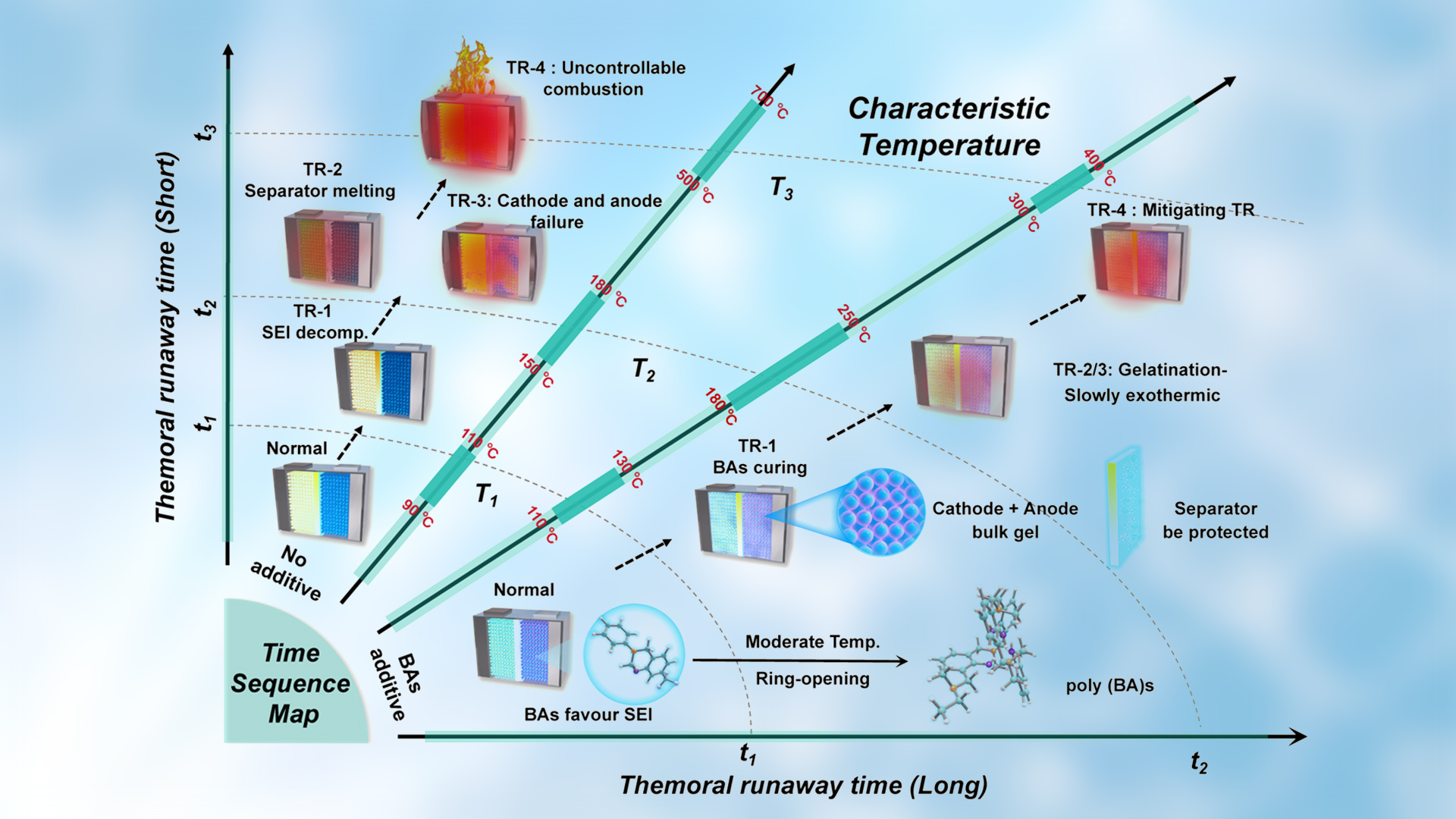
Figure 1. Schematic illustration of mitigating TR design
To overcome this, a research team led by Professor Yonghong Deng from the Department of Materials Science and Engineering at the Southern University of Science and Technology (SUSTech), in collaboration with Dr. Kang Xu from SES AI Corporation, has introduced a novel full-lifecycle, smart thermoresponsive electrolyte additive: 3-phenyl-7-(trifluoromethyl)-3,4-dihydro-2H-1,3-benzoxazine (mCF₃-BA).
Their paper, entitled “A thermoresponsive electrolyte additive for high-energy, long-cycling, and safe lithium batteries,” has been published in Joule, a distinctive and forward-looking journal in the field of energy. This work has attracted widespread attention within the industry since its publication, with multiple companies expressing their interest in collaboration.
The researchers introduced a simple yet powerful safety-enhancing strategy: instead of redesigning the entire electrolyte system, they employ a precisely engineered additive that shifts its role between normal and abusive conditions. This approach effectively unites high energy density with robust safety, overcoming the long-standing trade-off between performance and protection.
Specifically, they employed 3-phenyl-3,4-dihydro-2H-1,3-benzoxazine (BA) and 3-phenyl-7-(trifluoromethyl)-3,4-dihydro-2H-1,3-benzoxazine (mCF3-BA) as thermoresponsive additives for electrolytes to mitigate TR over the full scale of failure mechanisms. Owing to the robust SEI formed by mCF3-BA and the fast polymerization at threshold temperature, a balance was found between electrochemical performance and safety.
The working mechanism of the thermoresponsive additive (mCF3-BA) when subjected to TR involves a two-stage process (Figure 1). Part of the mCF3-BA polymerizes at the electrode to form poly(mCF3-BA), which facilitates the formation of homogeneous, LiF-enriched, and temperature-resistant cathode electrolyte interphase (CEI)/SEI. Such interphases raise the onset temperature T1 from 85.3°C to 103.5°C for a Li||NCM811 battery, and from 105.7°C to 119.3°C for a Si@Gr450 (a mixture of silicon and graphite with a designated capacity of 450 mAh g−1)||NCM811 battery.
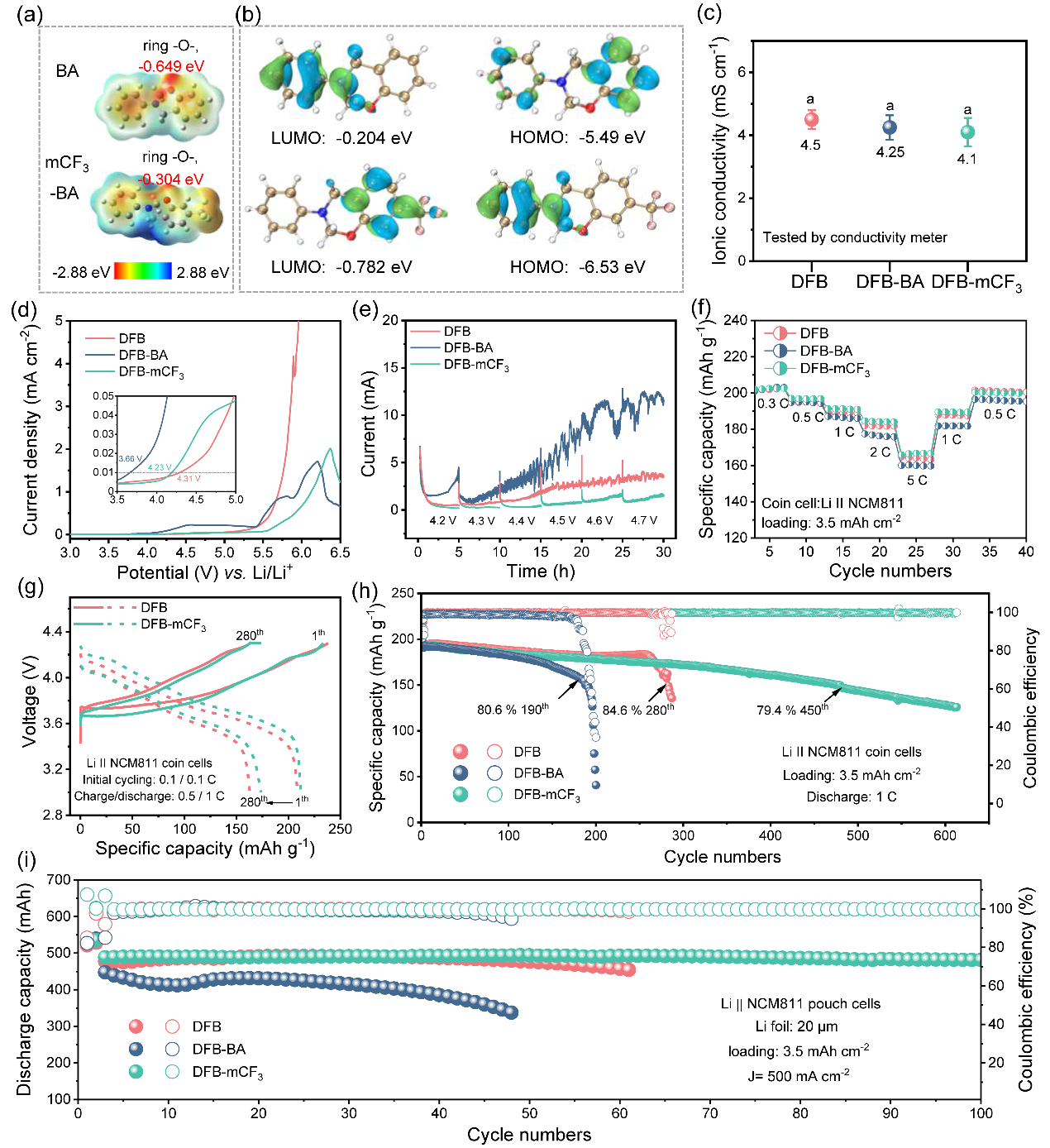
Figure 2. Electrochemical stability and performance of BA/mCF3-BA additives in Li||NCM811 cells
As the temperature continues to increase, the residual mCF3-BA additive undergoes rapid polymerization, forming an insulating thermoset resin within the cells. The resin effectively inhibits chemical crosstalk and electrochemical reactions between anode and cathode materials, raising T2 from 153.1°C to 187.6°C for Li||NCM811, and from 142.8°C to 186.7°C for Si@Gr450||NCM811.
This work presents a promising pathway for future high-energy-density and safe batteries for practical applications.
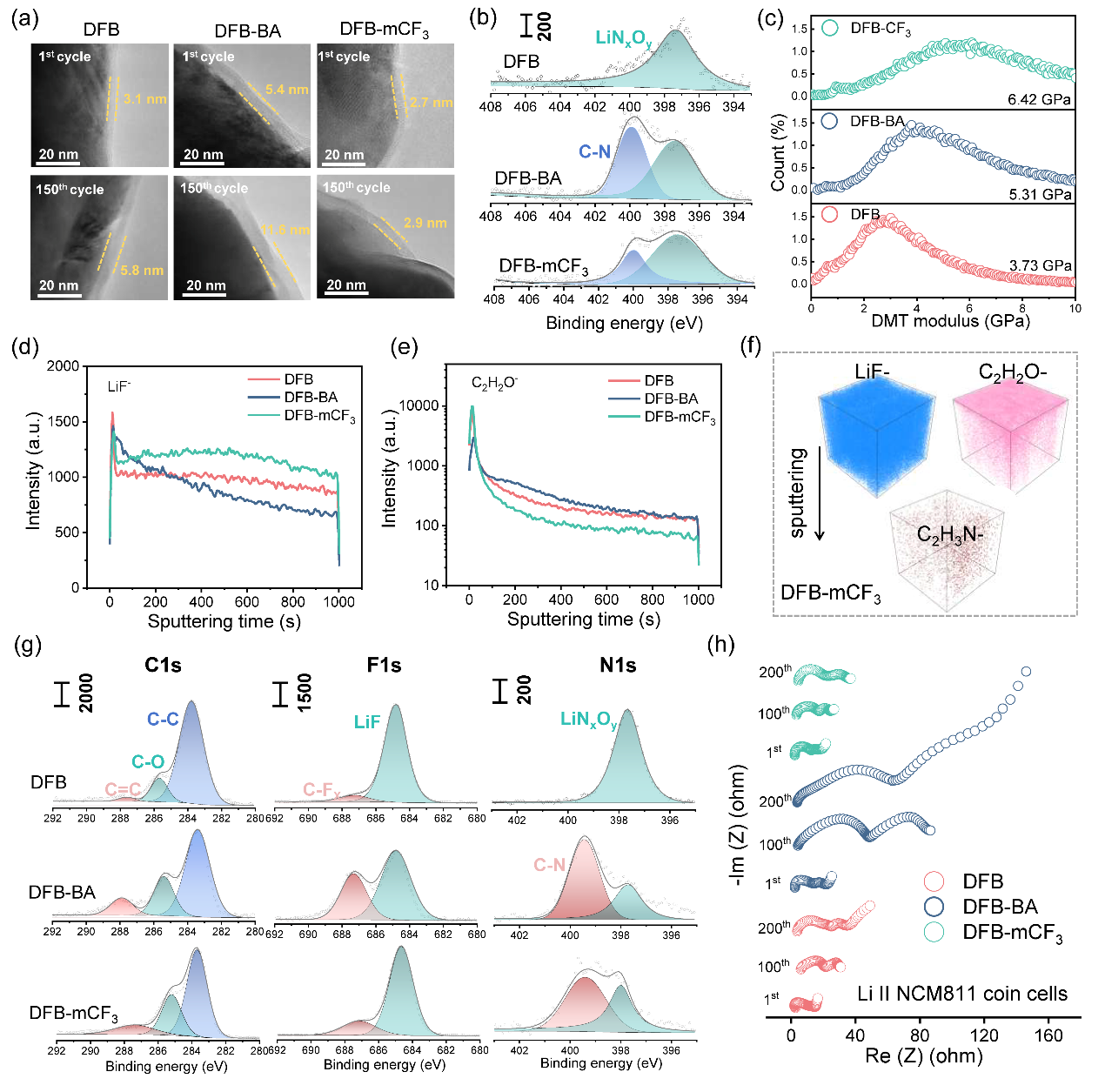
Figure 3. Characterizations on SEI and CEI of Li||NCM811 cells
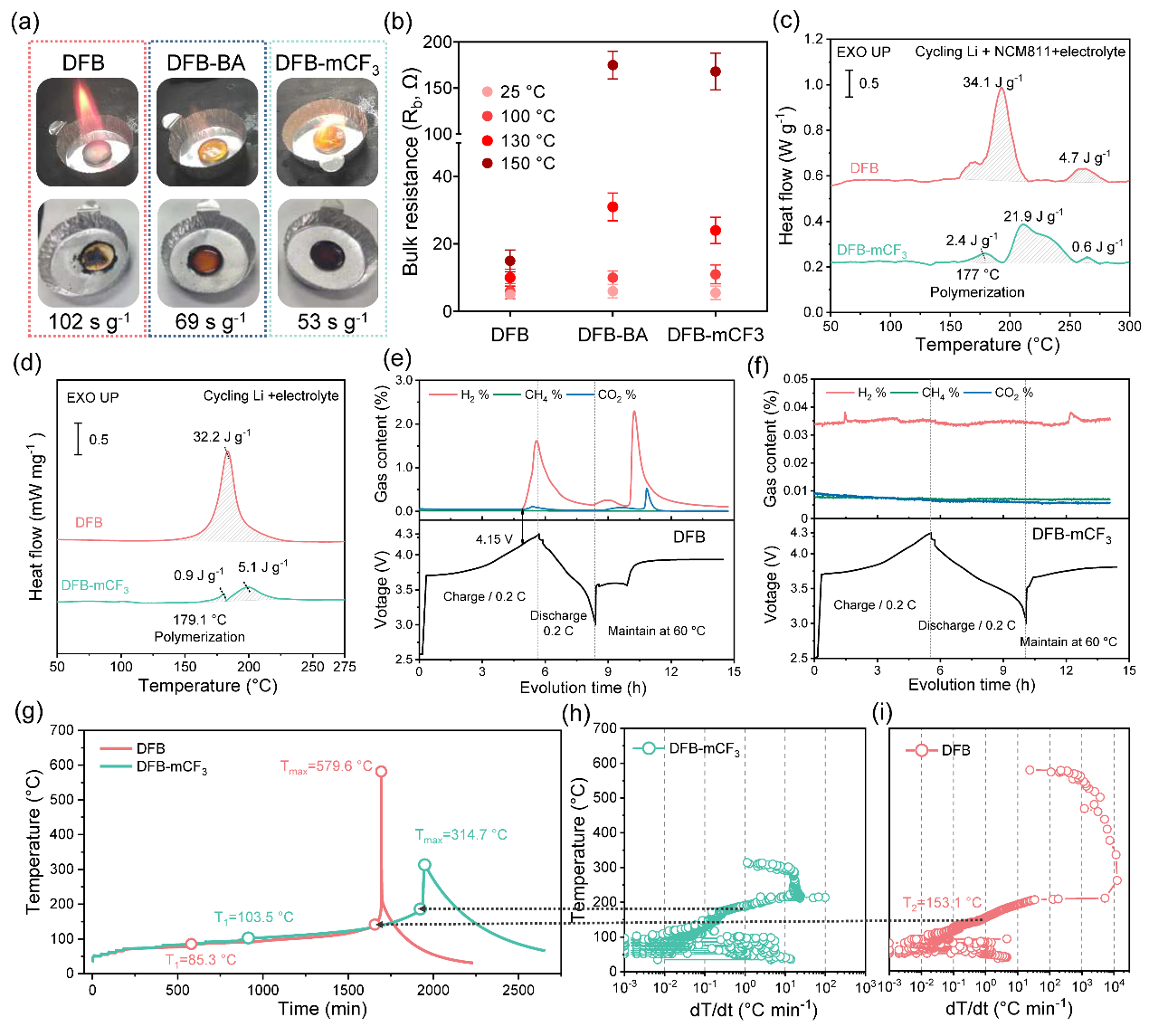
Figure 4. Assessment of TR-mitigation effects in 500 mAh Li||NCM811 pouch cells
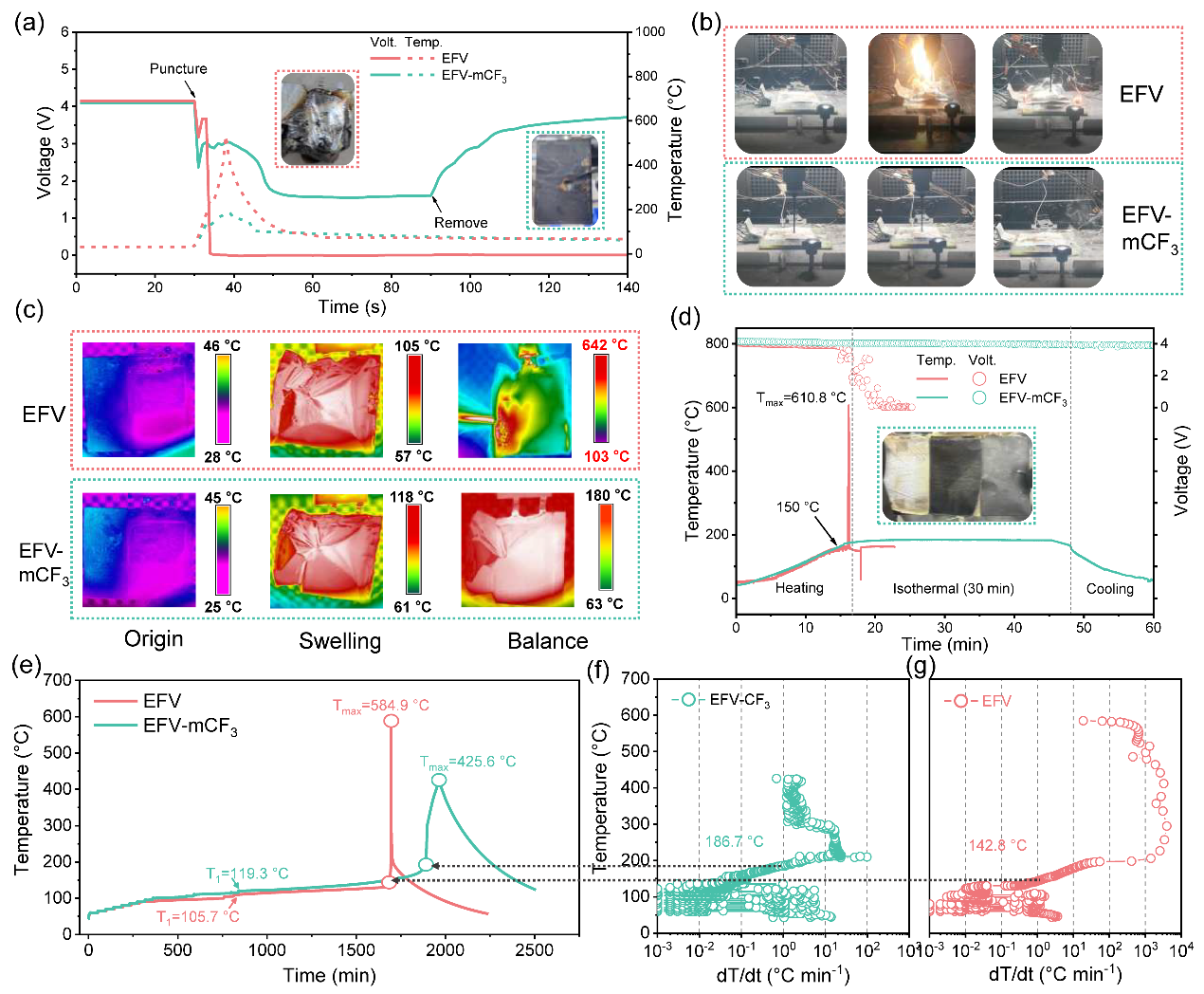
Figure 5. Safety assessments of 1,800 mAh Si@Gr450||NCM811 pouch cells
The co-first authors of the paper are Ph.D. students Yong Zeng and Fangzheng Liu. The co-corresponding authors are Professor Yonghong Deng, Dr. Kang Xu, along with Research Professor Jun Wang and Associate Researcher Hongli Xu from SUSTech. Collaborating institutions include South China University of Technology and the Hong Kong University of Science and Technology.
Paper link: https://doi.org/10.1016/j.joule.2025.102100
To read all stories about SUSTech science, subscribe to the monthly SUSTech Newsletter.
Proofread ByAdrian Cremin, Yilin ZHOU
Photo ByDepartment of Materials Science and Engineering