Quan LIU’s team at the School of Medicine published a study in the American Journal of Transplantation titled “TMEM16F-CLIC1 Interaction Mediates Recipient DC Cross-Decoration After Transplantation.” The study reveals the pivotal role of the TMEM16F-CLIC1 interaction in the “cross-decoration” of recipient dendritic cells (DCs) following transplantation and investigates the potential of niclosamide as a donor-specific immunosuppressive agent for transplant rejection.
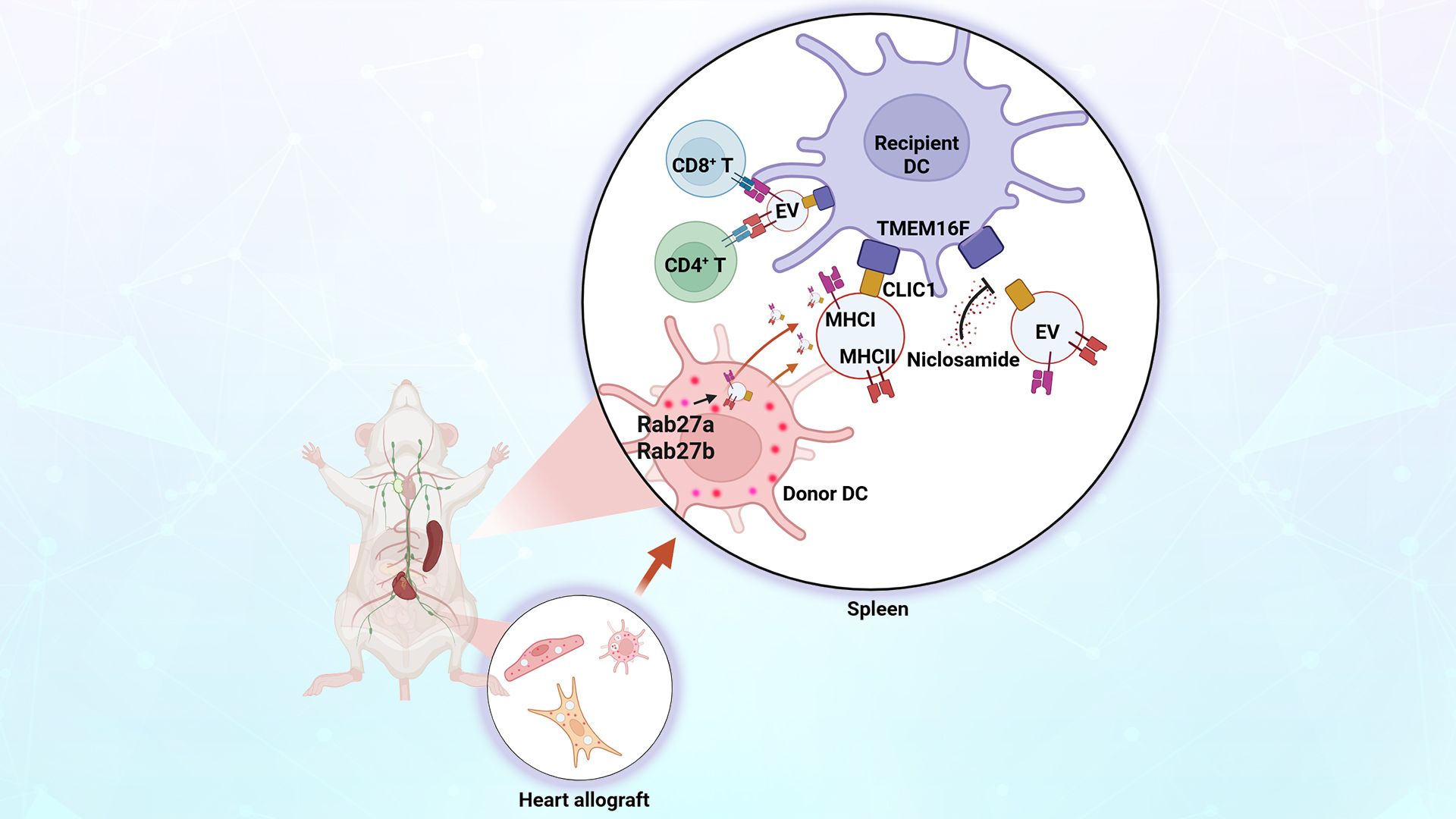
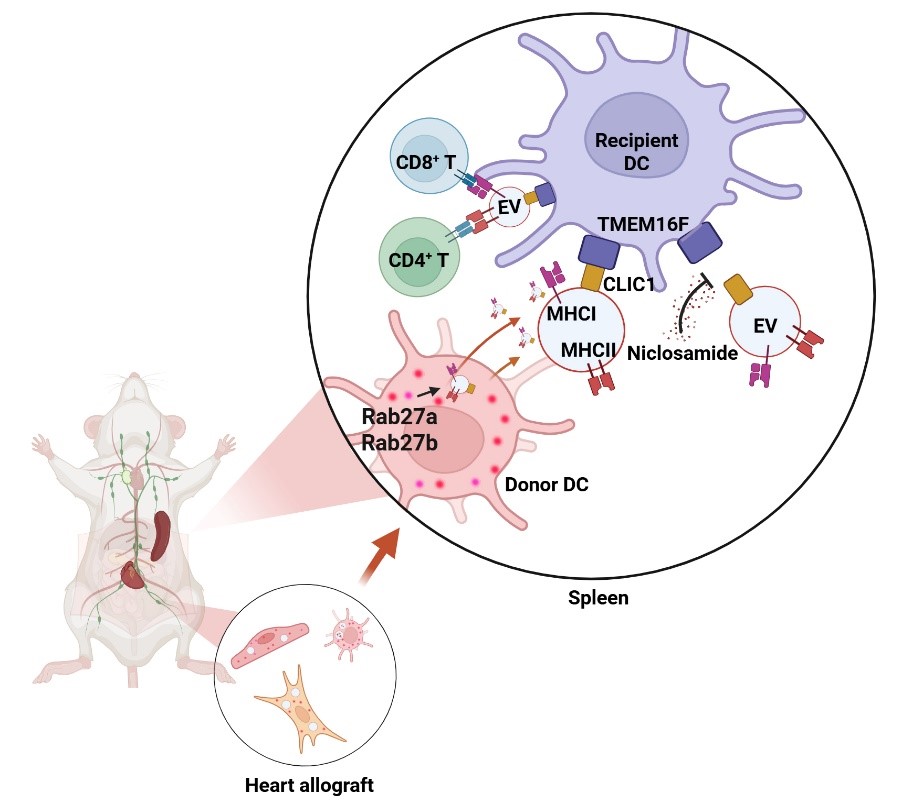
Allogeneic transplant rejection remains a leading cause of graft failure. Current immunosuppressive therapies, which lack antigen specificity, often result in significant side effects, including an increased risk of tumors and infections. To address this challenge, a deeper understanding of how transplant antigens are presented is critical for developing donor-specific immunosuppressive treatments. Transplant rejection is primarily initiated when the recipient’s immune system recognizes and responds to donor major histocompatibility complex (MHC) molecules. Traditional dogma suggests that donor DCs directly activate recipient T cells through MHC molecules, triggering an acute rejection response. However, Quan LIU challenged this theory, proposing instead that extracellular vesicles (EVs) from donor DCs, carrying MHC molecules, cross-decorate recipient DCs. This discovery introduces a novel semi-direct pathway for transplant antigen presentation driven by the “cross-decoration” of recipient DCs.
Recently, LIU’s team found that reducing EV production from donor DCs could mitigate rejection in cardiac transplants and extend graft survival. Further proteomic and molecular analysis revealed that the interaction between the membrane proteins TMEM16F and CLIC1 is essential for facilitating the binding of donor EVs to recipient DCs. The researchers also discovered that EVs released from endothelial cells in the graft can cross-decorate recipient DCs, promoting acute rejection, which clarifies the origin of donor EVs after the elimination of donor DCs. Based on these findings, the researchers applied an existing clinical anthelmintic drug, niclosamide, which specifically targets TMEM16F, to block the TMEM16F-CLIC1 interaction. This intervention effectively inhibited the binding of donor EVs to recipient DCs, limited cross-decoration, and suppressed acute rejection in heart transplantation. This novel approach offers a promising strategy for donor-specific immunosuppression, and the repurposing of niclosamide has already been granted a Chinese invention patent.
PhD student Xiaoshi LI and assistant professor Zhirong ZHANG are co-first authors of the paper. Associate Professor Quan LIU is the corresponding author. SUSTech is the primary corresponding institution.
Paper Link: https://www.sciencedirect.com/science/article/abs/pii/S1600613525031909
Proofread ByNoah Crockett, Junxi KE
Photo ByYan QIU