Recently, the research team led by Professor Lei XI from the Department of Biomedical Engineering at Southern University of Science and Technology (SUSTech) has successfully developed a Photoacoustic Computed Mesoscopy (PACMes) system, which achieves label-free, long-term, and high-resolution visualization of the mouse cerebral cortical vascular network through the intact scalp and skull. The relevant results were published in Science Advances under the title “Noninvasive Photoacoustic Computed Mesoscopy for Longitudinal Brain Imaging”.
Non-invasive long-term brain imaging technology is crucial for deciphering brain physiological functions and exploring the pathological mechanisms of brain diseases. It can maximize the preservation of the animal’s native physiological state and realize dynamic monitoring of the entire disease process. However, complex structures such as the scalp and skull of the mouse brain cause light refraction, strong scattering, and ultrasonic attenuation, which seriously affect the imaging effect. In existing technologies, improving resolution often requires invasive operations such as scalp removal, skull thinning, or even cranial window implantation, which are prone to side effects such as changes in intracranial pressure and mechanical damage. In contrast, non-invasive imaging technologies such as near-infrared II region fluorescence imaging have problems of fluorescence photobleaching and metabolic clearance; ultrasound localization microscopy requires repeated injection of microbubble contrast agents; and photoacoustic computed tomography is limited by the diffraction limit of low-frequency ultrasound, making it difficult to achieve long-term, non-invasive, and high-resolution imaging.
The research team proposed a new PACMes imaging strategy, constructing an integrated non-invasive photoacoustic imaging system through coordinated optimization of three dimensions: near-infrared optical excitation, low-frequency acoustic detection, and computational reconstruction. This system innovatively integrates multi-angle scanning of near-infrared focused line light, a 5MHz low-frequency full-ring ultrasound transducer array, and a composite reconstruction algorithm. By using near-infrared focused line light to maintain high optical resolution in the direction perpendicular to the length of the line light while maximizing excitation efficiency, it achieves efficient penetration through the intact scalp and skull and reduces light scattering interference. Meanwhile, the low-frequency full-ring transducer array realizes high-sensitivity full-view detection of photoacoustic signals, greatly reducing the impact of acoustic attenuation in biological tissues. Through the development of a composite computational reconstruction algorithm combining filtered back projection and optical localization of photoacoustic signals, the team not only broke through the diffraction limit of low-frequency ultrasound in the direction of the length of the parallel line light but also effectively suppressed background artifacts, ultimately achieving isotropic high-resolution imaging in the entire field of view. System tests show that PACMes can image a 13mm diameter field of view (covering the entire mouse cerebral cortex) through the intact scalp and skull without exogenous contrast agents, with a spatial resolution of 33μm, and can support continuous long-term monitoring for more than 5 months. The team also designed a dual model of SOS calibration and coordinate calibration, combined with a deep learning self-supervised recovery algorithm, reducing the required scanning angles from 180 to 18, achieving a 10-fold improvement in imaging speed, while effectively suppressing artifacts caused by tissue sound speed mismatch and improving the structural fidelity of images.
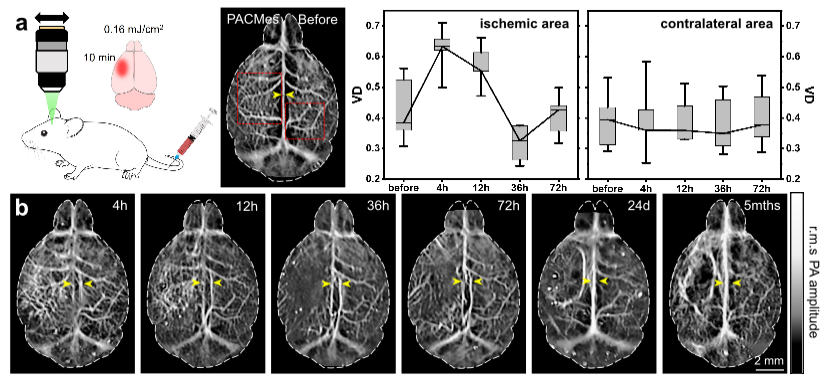
Fig. 1 Long-term monitoring of the cerebral vasculature in photothrombosis-induced ischemic stroke.
The research team conducted long-term imaging studies on mice with two classic brain disease models: stroke and glioma. In the mild ischemic stroke model, the team used this system to achieve dynamic monitoring for more than 5 months, clearly capturing the complete change process of vascular density in the infarct area, which first increased, then decreased, and finally returned to the baseline. They also observed the key pathological feature of new collateral circulation in the infarct area 72 hours after modeling under non-invasive conditions, providing direct visual evidence for the study of vascular repair mechanisms after stroke. In the glioma model, the PACMes system successfully tracked typical pathological manifestations such as vascular morphological distortion and sagittal sinus compression during tumor growth. Its imaging results were highly consistent with those of magnetic resonance imaging (MRI) and histopathological staining, realizing non-invasive full-course monitoring of tumor vascular remodeling.
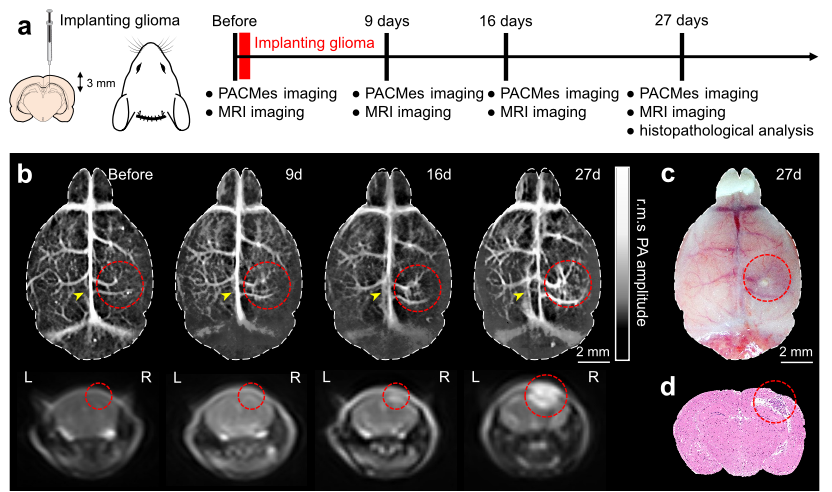
Fig. 2 Long-term monitoring of the glioma inside the mouse brain.
The core advantage of this study is the realization of non-invasive, label-free, high-resolution long-term cerebral cortical vascular imaging. Compared with existing technologies, it does not require invasive operations on experimental animals or injection of exogenous contrast agents, avoiding the impact of contrast agent metabolic burden and photobleaching on long-term imaging, and providing an ideal tool for monitoring the chronic course of brain diseases. In the future, combined with objectives with higher numerical aperture and multispectral excitation strategies, PACMes technology can also achieve whole-brain functional imaging at the capillary level, and further expand to multi-parameter brain metabolism monitoring such as blood oxygen and lipids, opening up a new technical path for the study of pathological mechanisms and therapeutic effect evaluation of more cerebrovascular-related diseases such as Alzheimer’s disease and epilepsy.
SUSTech is the first and corresponding affiliation of the paper. Shijie RUAN, Wei Qin , and Linyang LI from the Department of Biomedical Engineering are co-first authors, and Professor Lei XI is the corresponding author.
Paper link: https://www.science.org/doi/10.1126/sciadv.aea1602
Proofread ByNoah Crockett, Junxi KE
Photo ByShijie Ruan