Assistant Professor Xin HONG at the Department of Biochemistry, SUSTech Homeostatic Medicine Institute, School of Medicine, Southern University of Science and Technology (SUSTech), in collaboration with Associate Chief Physician Dr. Weinan GUO from Xijing Hospital of Air Force Medical University and Professor Hao Yu from the Shenzhen Institute of Advanced Technology of Chinese Academy of Sciences, published a research article online in the prestigious international cancer journal Cancer Research. The paper is titled “Cortactin Suppresses mTOR-Dependent Senescence in Circulating Tumor Cells.”
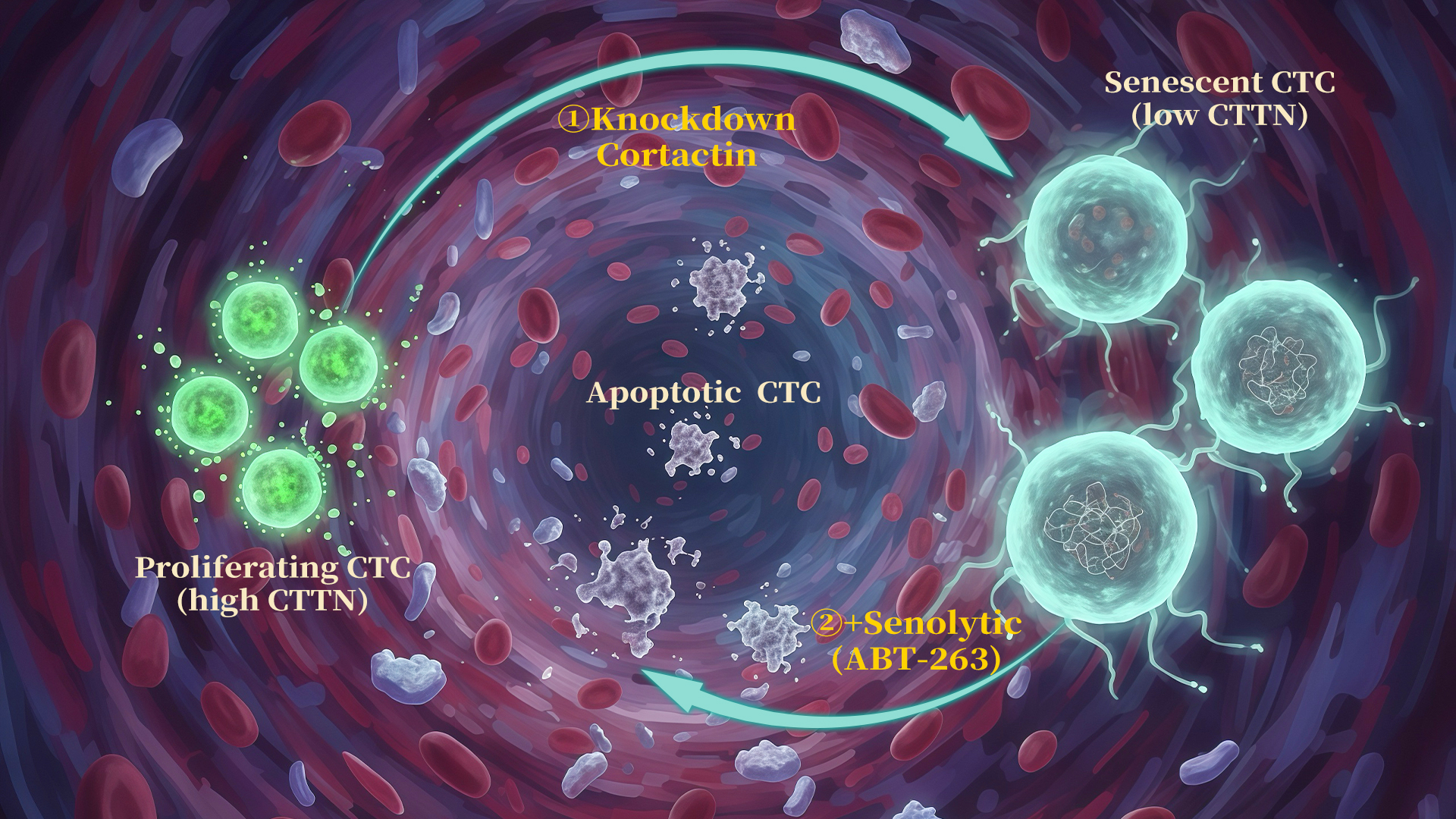
This study reveals for the first time that Cortactin—a cytoskeletal regulatory protein—enables melanoma circulating tumor cells (CTCs) to maintain late endosome homeostasis, thereby suppressing an mTOR/p53-dependent senescence program. This mechanism allows cancer cells to survive the highly stressful environment of the bloodstream and accomplish distant metastasis. Importantly, the research team proposed an innovative strategy, “senescence induction followed by senolytic therapy,” which significantly inhibits hematogenous metastasis.
Circulating tumor cells (CTCs) are “metastatic seeds” shed from primary tumors into the bloodstream; however, the vast majority die due to oxidative stress, immune attack, or cellular senescence. This study found that, compared with CTCs from other cancer types, melanoma CTCs exhibit high expression of Cortactin, which is negatively correlated with overall survival (OS) and distant metastasis-free survival (DMFS) in melanoma patients. Knockdown of Cortactin significantly induced senescence in patient-derived melanoma CTC cell lines, as evidenced by activation of the p53/p21 pathway, G0/G1 cell cycle arrest, upregulation of the senescence-associated secretory phenotype (SASP) gene signature, positive SA-β-gal staining, and decreased expression of Ki-67 and lamin B1, accompanied by a marked increase in mitochondrial reactive oxygen species (mtROS) (Figure 1). Notably, a high SASP signature was associated with improved OS in patients (Figure 1). Further mechanistic investigations revealed that Cortactin localizes to late endosomes and is essential for maintaining their structural and functional homeostasis. Cortactin knockdown caused abnormal aggregation of late endosomes, which recruited and activated mTOR, leading to phosphorylation and activation of p53 and ultimately triggering p53-dependent senescence in CTCs. Treatment with various mTOR inhibitors effectively suppressed the p53/p21 signaling axis and attenuated the senescent phenotype (Figure 2). Additionally, the study uncovered a positive feedback loop between p53 and mtROS, which is critical for stabilizing the senescent state of CTCs (Figure 4).
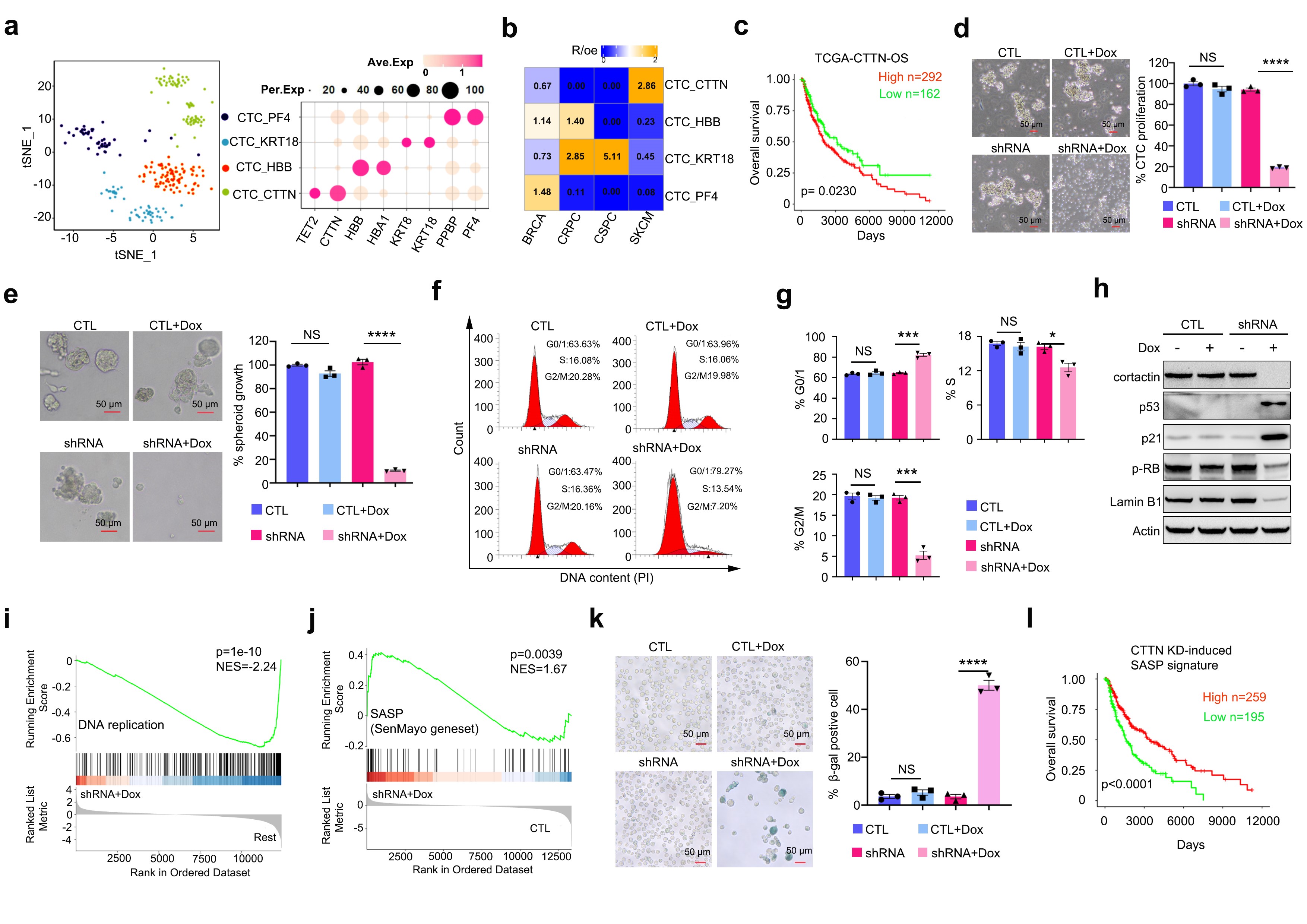
Figure 1. Cortactin knockdown induces senescence in melanoma circulating tumor cells (CTCs).
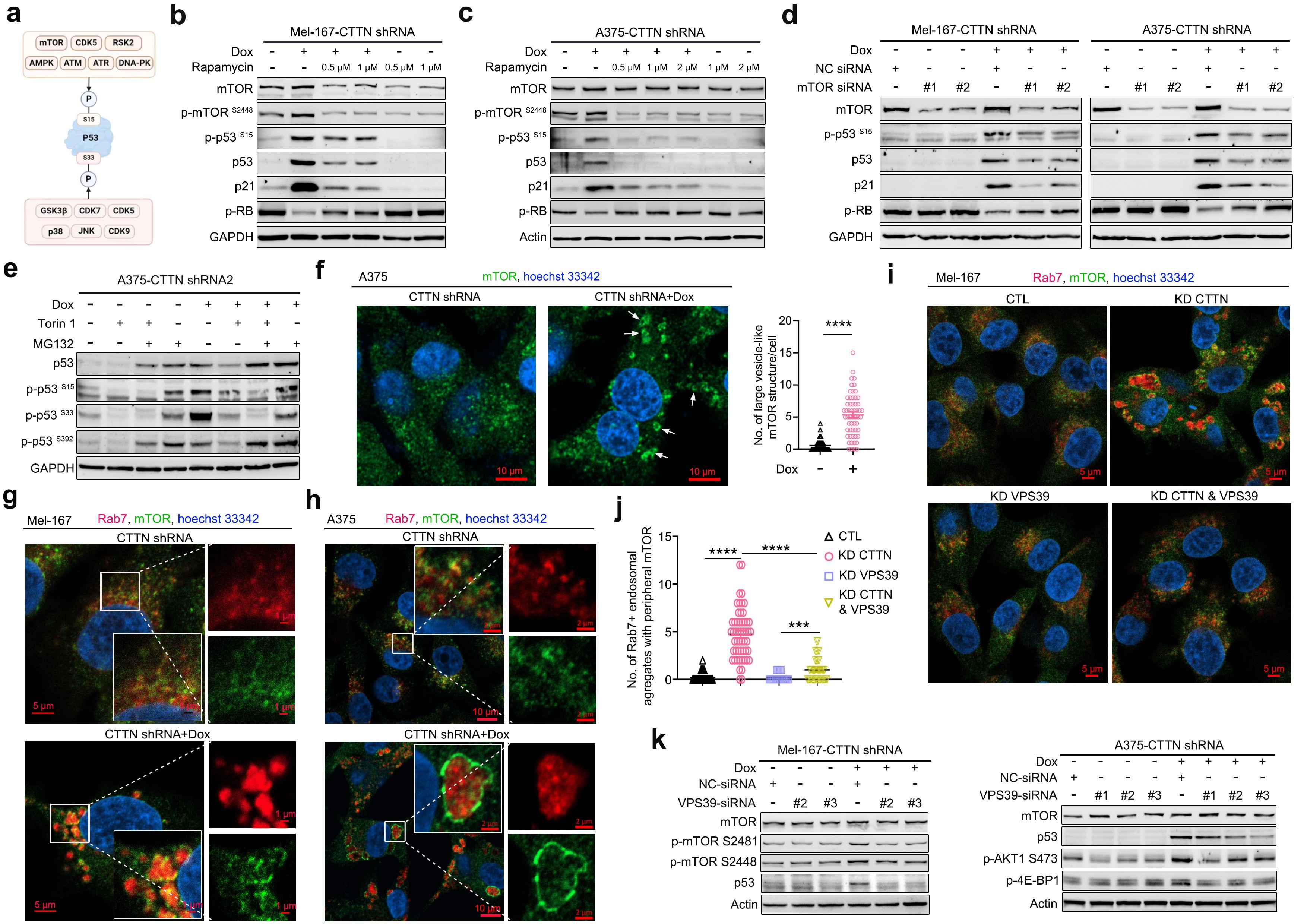
Figure 2. Cortactin knockdown leads to late endosome aggregation and activation of the mTOR/p53 signaling pathway.
Cortactin knockdown-induced senescence in CTCs is characterized by a high expression of the BCL2L1 gene (encoding Bcl-xl), suggesting that these senescent cells may utilize the elevated expression of this gene to exert anti-apoptotic effects and maintain cell survival. Based on this observation, the research team devised a “one-two punch” sequential therapy: firstly, inducing senescence in CTCs by inhibiting Cortactin function, followed by selectively eliminating these senescent CTCs using the Bcl-xL inhibitor ABT-263 (acting as a senolytic drug). This strategy significantly reduced the number of CTCs and effectively inhibited hematogenous metastasis in animal models (Figure 3).
In a prospective cohort of melanoma patients, the increased proportion of SA-β-gal positive senescent CTCs in peripheral blood was significantly associated with treatment resistance and disease progression, indicating that these “dormant but not dead” senescent CTCs could be a potential risk factor for tumor recurrence and metastasis (Figure 3 p-r).
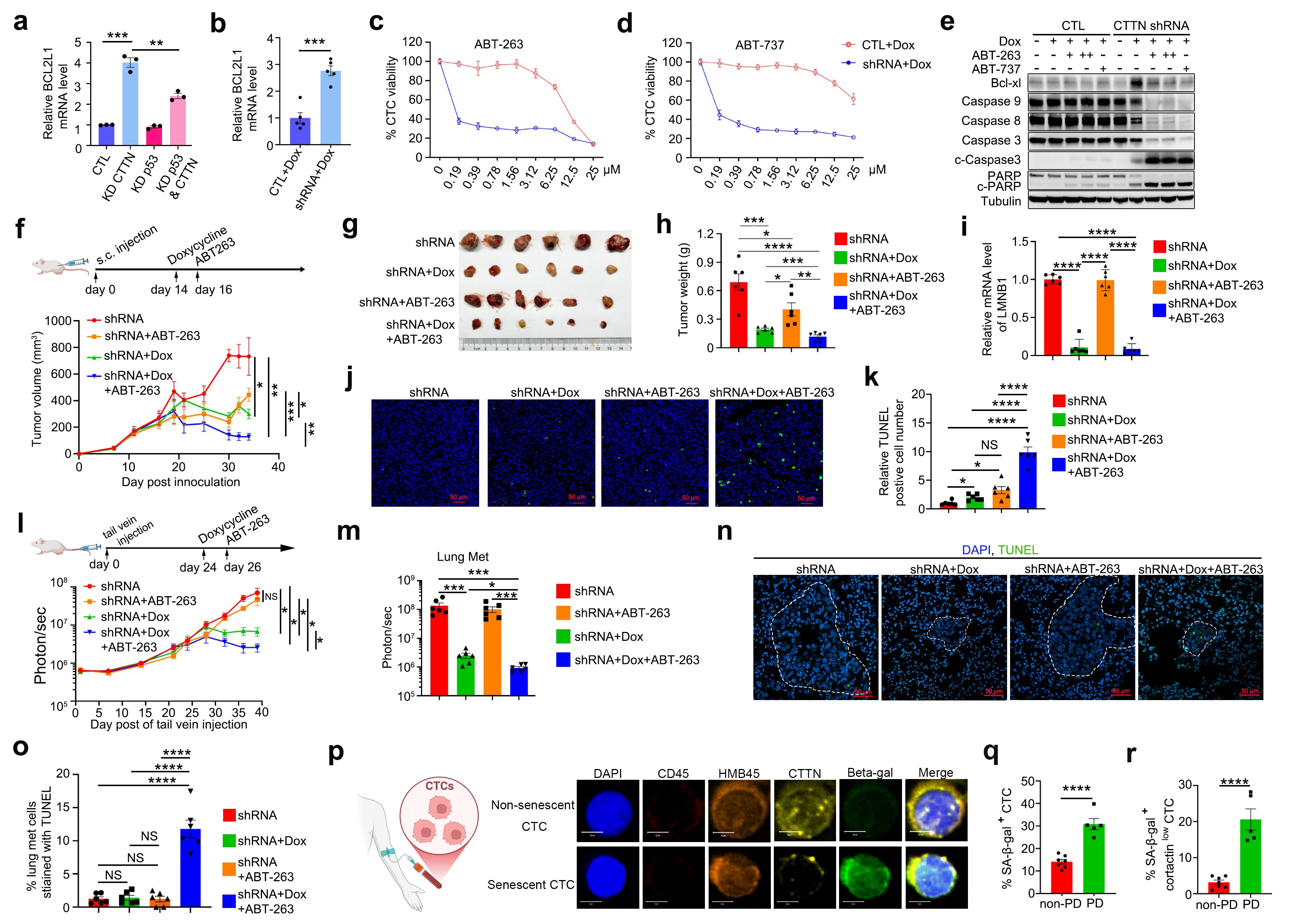
Figure 3. Sequential therapy of “Cortactin knockdown-induced senescence + senolytic clearance” suppresses tumor growth and metastasis of melanoma CTCs
This study not only uncovers the central role of the Cortactin/mTOR/p53/mtROS axis in regulating the fate of circulating tumor cells (CTCs) but also proposes a novel anti-metastatic strategy, “induce senescence first, then precisely eliminate senescent cells” (Figure 4). These findings provide a crucial theoretical foundation and potential therapeutic targets for developing new interventions specifically aimed at CTCs.
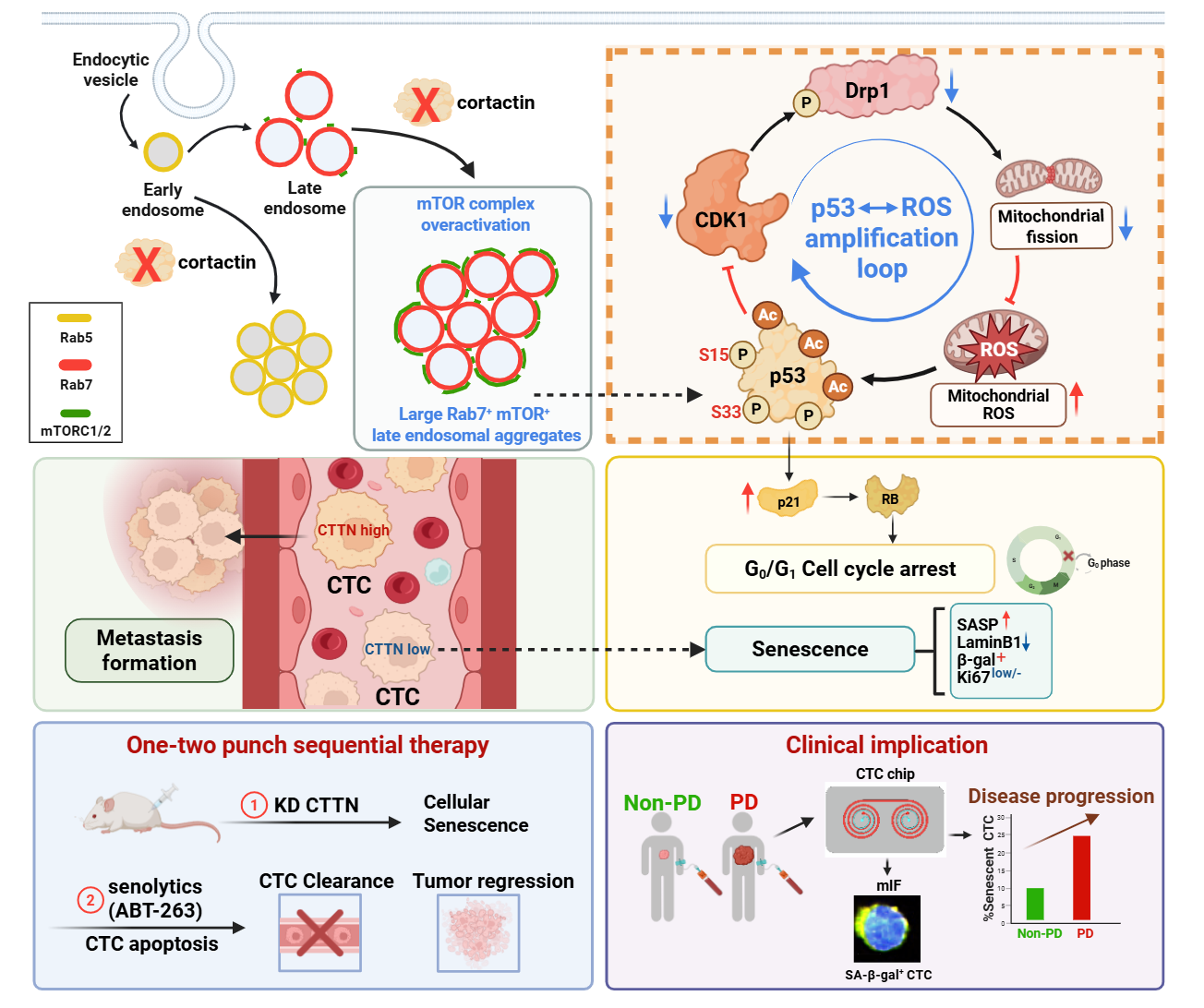
Figure 4. Illustration of the Cortactin/mTOR/p53/mtROS signaling pathway axis in the regulation of senescence in melanoma CTCs and the sequential therapy approach.
Dr. Jianyang HU, Research Assistant Professor at SUSTech; Binyu ZHANG, a 2024 joint doctoral student co-supervised by SUSTech and Capital Medical University; Guanyin HUANG, a 2025 Ph.D. student at SUSTech; and Junhao CHEN, a 2022 Ph.D. student at Jinan University (visiting student at SUSTech), are co-first authors of this paper. Assistant Professor Xin HONG at the Department of Biochemistry, SUSTech Homeostatic Medicine Institute, School of Medicine at SUSTech; Associate Chief Physician Dr. Weinan GUO from Xijing Hospital, Air Force Medical University; and Professor Hao YU from the Shenzhen Institute of Advanced Technology, Chinese Academy of Sciences, are co-corresponding authors. SUSTech is the institution for the first author and the last corresponding author of this paper.
Paper Link:https://pubmed.ncbi.nlm.nih.gov/41418100/ (DOI: 10.1158/0008-5472.CAN-25-1175)
Proofread ByNoah Crockett, Junxi KE
Photo ByYan QIU