A research team, led by Assistant Professor Yang LEI, in the School of Environmental Science and Engineering at Southern University of Science and Technology (SUSTech), has revealed the mechanism behind surface passivation in conventional lime-based precipitation and proposed an innovative spatially decoupled electrochemical technique to overcome lime passivation and achieve efficient phosphorus (P) recovery. The paper with their findings, entitled “Spatially decoupled electrochemical strategy for lime passivation prevention and sustainable phosphate recovery,” has been published in Nature Communications.

Lime-based precipitation, though widely adopted for wastewater P removal, suffers from surface passivation. The passivation layer inhibits Ca2+ release, forcing excessive dosing while yielding low-quality sludge and effluent with elevated hardness and pH. Surface passivation, a universal challenge prevalent in environmental geochemical systems, is plaguing processes reliant on solid-liquid reactions such as acidic mine drainage remediation, zero-valent iron corrosion, and enhanced rock weathering. Thus, understanding passivation mechanisms and developing practical mitigation strategies are essential for advancing efficient and sustainable environmental remediation processes.
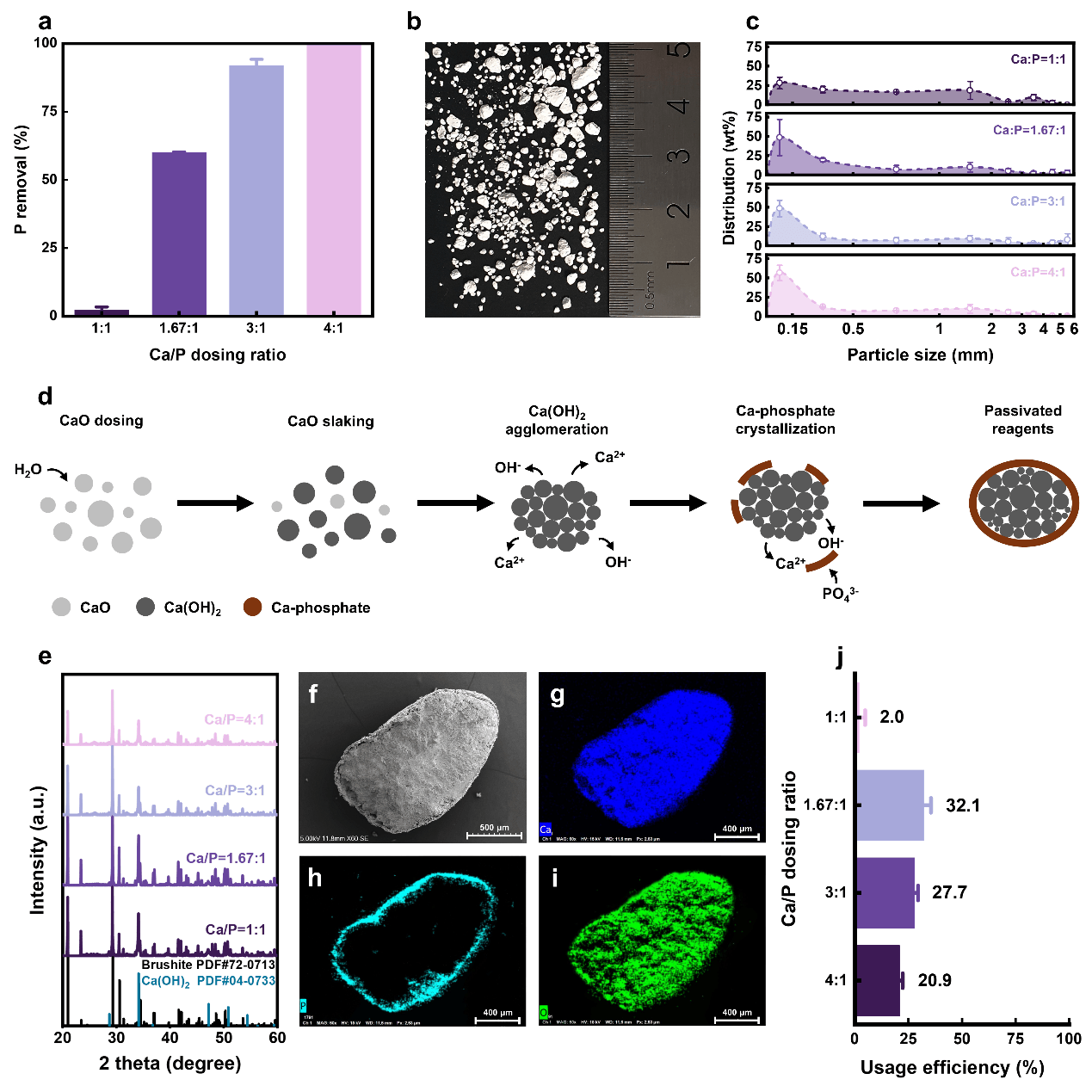
Fig. 1: Lime surface passivation and mechanism illustration
The team found that the substantial heat brought by lime hydration dramatically decreases Ca(OH)₂ solubility, promoting rapid Ca(OH)2 agglomeration. In wastewater, Ca²⁺ and OH⁻ was released from these agglomerates and combine with phosphates, forming a dense Ca-phosphate passivation layer on the particle surface, inhibiting further Ca2+ release and P removal (Fig. 1). The lime passivation, inversely governed by Ca(OH)2 solubility, necessitates substantial over-dosing (up to Ca/P ratio of 4:1) (Fig.1), ultimately yielding low-purity products (<6.9wt% P) and poor effluent quality (936.7mg L–1 Ca2+ and pH 12.7). While limestone presents an economically and environmentally favorable alternative, its efficacy remains critically limited (0.3–11.0% at Ca/P 1:1–4:1) due to rapid Ca-phosphate redissolution, underscoring the importance of stable alkalinity.
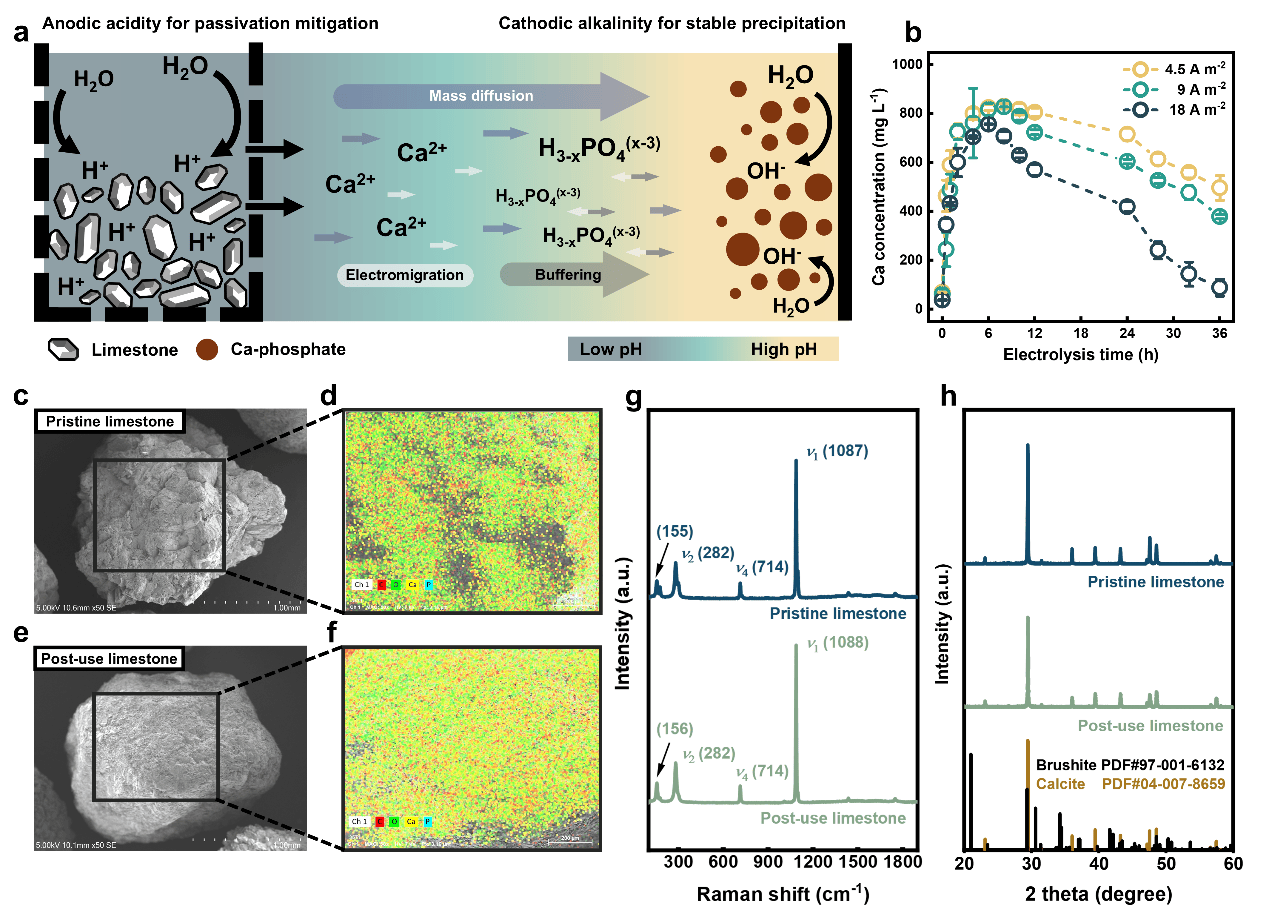
Fig. 2: Spatially decoupled electrochemical strategy tackling surface passivation and securing Ca2+ supply
The core challenge, whether in lime passivation or limestone precipitate redissolution, lies in the inseparable coupling of two key reactions within the same region: Ca2+ release (requiring acidity) and phosphate precipitation (requiring alkalinity).
Leveraging water electrolysis, the team developed an electrochemical strategy that spatially decouples the Ca2+ release and phosphate precipitation. By strategically positioning limestone in the acidic anode zone, sustained anti-passivation and efficient Ca2+ release were secured (Fig. 2). In the alkaline cathode zone, in situ-generated alkalinity drives Ca-phosphate precipitation, enabling efficient P recovery (85.7%). The system produces high-purity products (15.2 wt% P) at low energy consumption (14.8 kWh kg P-1) and delivers superior effluent quality (Fig. 3).
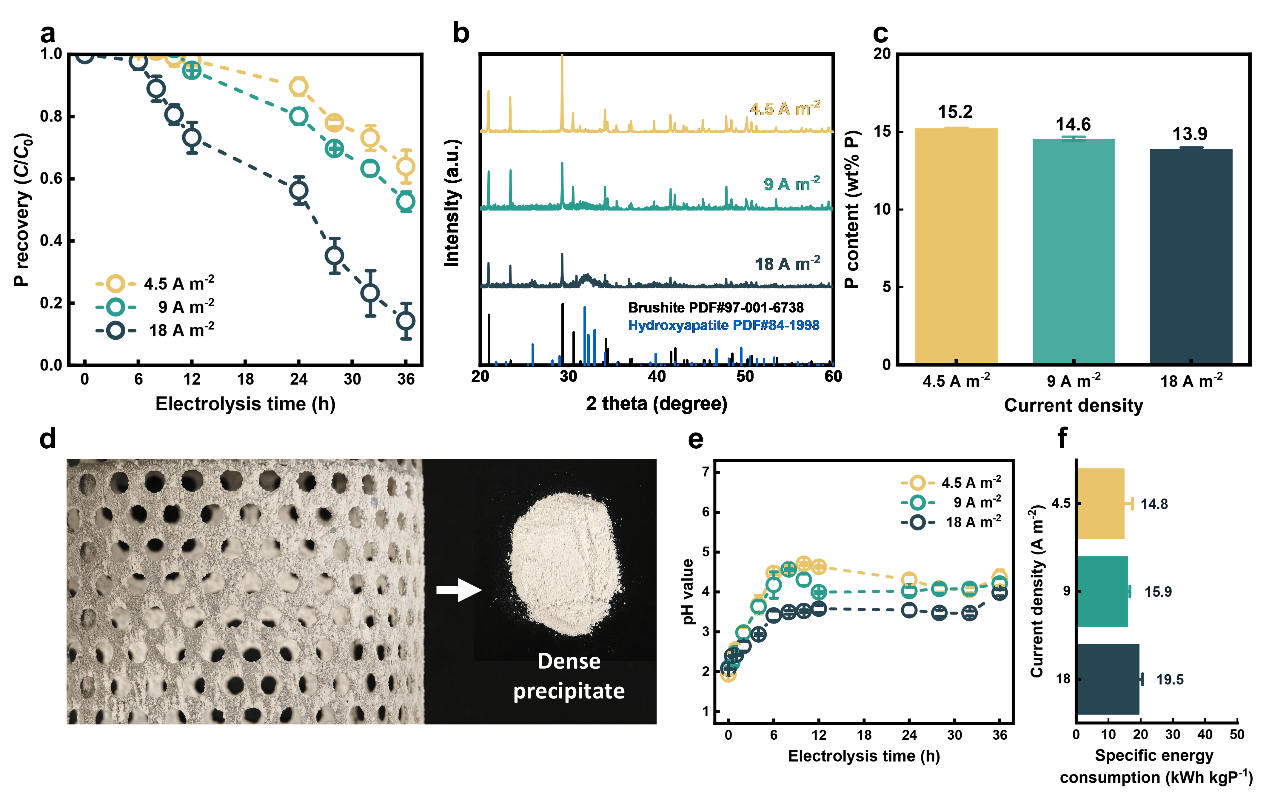
Fig. 3: The performance of the spatially decoupled electrochemical strategy in enhanced P recovery
Building upon mechanistic insights, the spatially decoupled electrochemical strategy demonstrates robustness and capacity flexibility over long-term operation, underscoring its implementation potential. The Techno-Economic Analysis (TEA) and Life Cycle Assessment (LCA) show that the electrochemical strategy can achieve 73.2% cost savings and a 29.1% reduction in carbon emissions by overcoming the inherent limitations of routine lime precipitation.
Ph.D. student Zhengshuo ZHAN and M.S. student Jingwen LV from the School of Environmental Science and Engineering are co-first authors of the paper. Assistant Professor Yang LEI is the corresponding author, with SUSTech serving as the first corresponding institution.
Paper Link: https://www.nature.com/articles/s41467-025-67911-1
Proofread ByNoah Crockett, Junxi KE
Photo ByYan QIU