Associate Professor Liu Leo LIU’s team from the Department of Chemistry at the Southern University of Science and Technology (SUSTech) has recently achieved the synthesis of a metal-free isodiazomethyl anion for the first time and elucidated its unique reactivity as a nitrene precursor. This groundbreaking research represents a significant advance in main-group and elemento-organic chemistry and was published in Nature Synthesis under the title “A crystalline isodiazomethyl anion.”
Basic organic motifs built from carbon and nitrogen are long-established cornerstones of synthetic chemistry. Over the past centuries, a diverse landscape of functional groups has been charted. Yet, it has become increasingly challenging to design new three-atom C/N assemblies with uncharted structures and properties. However, unconventional combinations of carbon and nitrogen atoms may still unlock previously unknown functional motifs with transformative potential for fundamental chemistry.
Since the seminal discovery of organic azides (Fig. 1a, R–N3, I) in the mid-19th century, these versatile species have become indispensable in chemistry and chemical biology, underpinning foundational transformations such as the Curtius rearrangement, Staudinger reduction, and “click” chemistry. Despite the rich diversity and widespread application of organic azides, their isoelectronic carbon analogues, diazomethyl anions (Fig. 1a, [R–CNN]–, II), are generally accessed as transient intermediates at low temperature. Recent breakthroughs in substituent design have enabled the synthesis and crystallographic characterization of certain diazomethyl anions. It was well-established that, upon nitrogen extrusion, organic azides and diazomethyl anions give rise to nitrenes (R–N) and carbyne anions [R–C]– respectively, which serve as pivotal synthons in organic transformations, functional materials, and other fields.
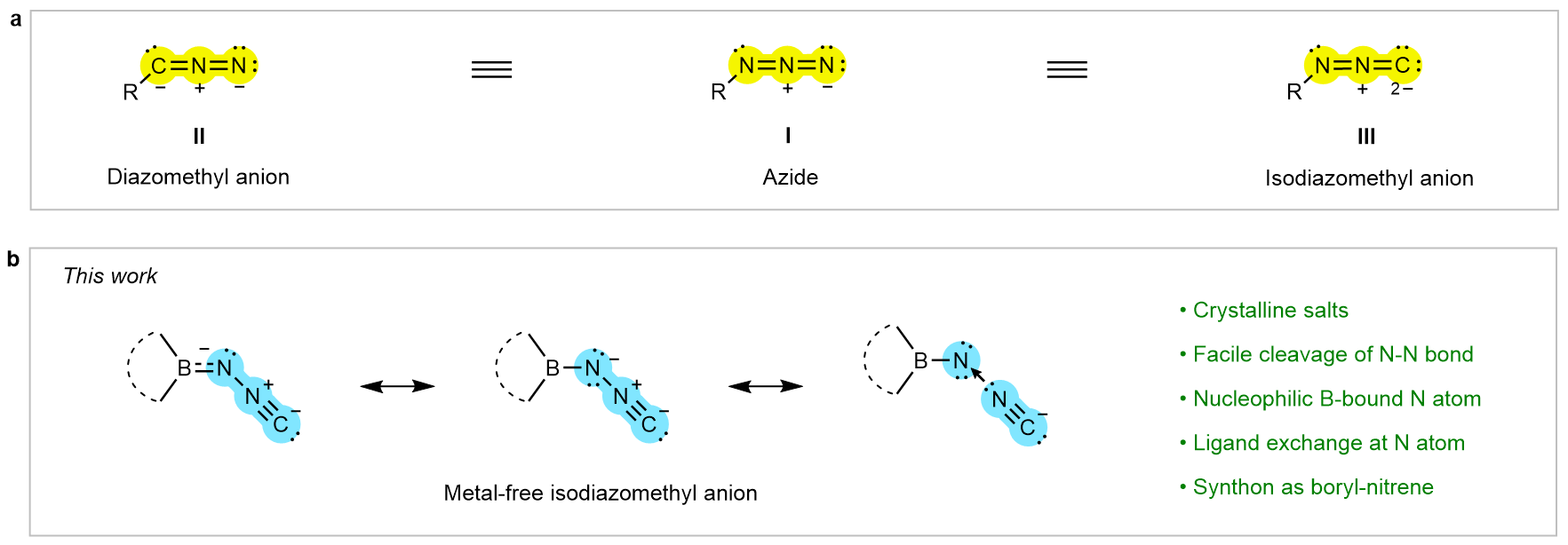
Figure 1: Background and scope of this work.
In sharp contrast, isodiazomethyl anions (Fig. 1a, [R–NNC]–, III) are exceedingly rare and have long posed a significant challenge in synthetic chemistry. Compared with II, isodiazomethyl anions exhibit substantially lower thermodynamic stability, rendering them inaccessible under ambient conditions. Although tautomerism between II and III was proposed in the early 20th century, only a few studies over the decades have reported in situ characterization of isodiazomethyl anions in solution, or accessed them through stabilization by transition-metal coordination. However, the isolation and structural authentication of a metal-free isodiazomethyl anion have not been reported to date, leaving its intrinsic electronic structure and reactivity essentially unexplored.
Ambiphilicity is a distinctive chemical property that enables a species to function as both a nucleophile and an electrophile. In recent years, the research group led by Liu Leo LIU has been dedicated to the study of ambiphilic main group chemistry. The isodiazomethyl anion [R–NNC]– can be regarded as an adduct of a nitrene [R–N] and a cyanide anion [NC]– (Fig. 1b), and its stable realization is expected to open new avenues in nitrene chemistry. Recently, the team achieved a metal-free isodiazomethyl anion by employing a bulky cyclic boryl substituent with π-accepting ability, thereby revealing a new class of nitrene precursors (Fig. 1b).
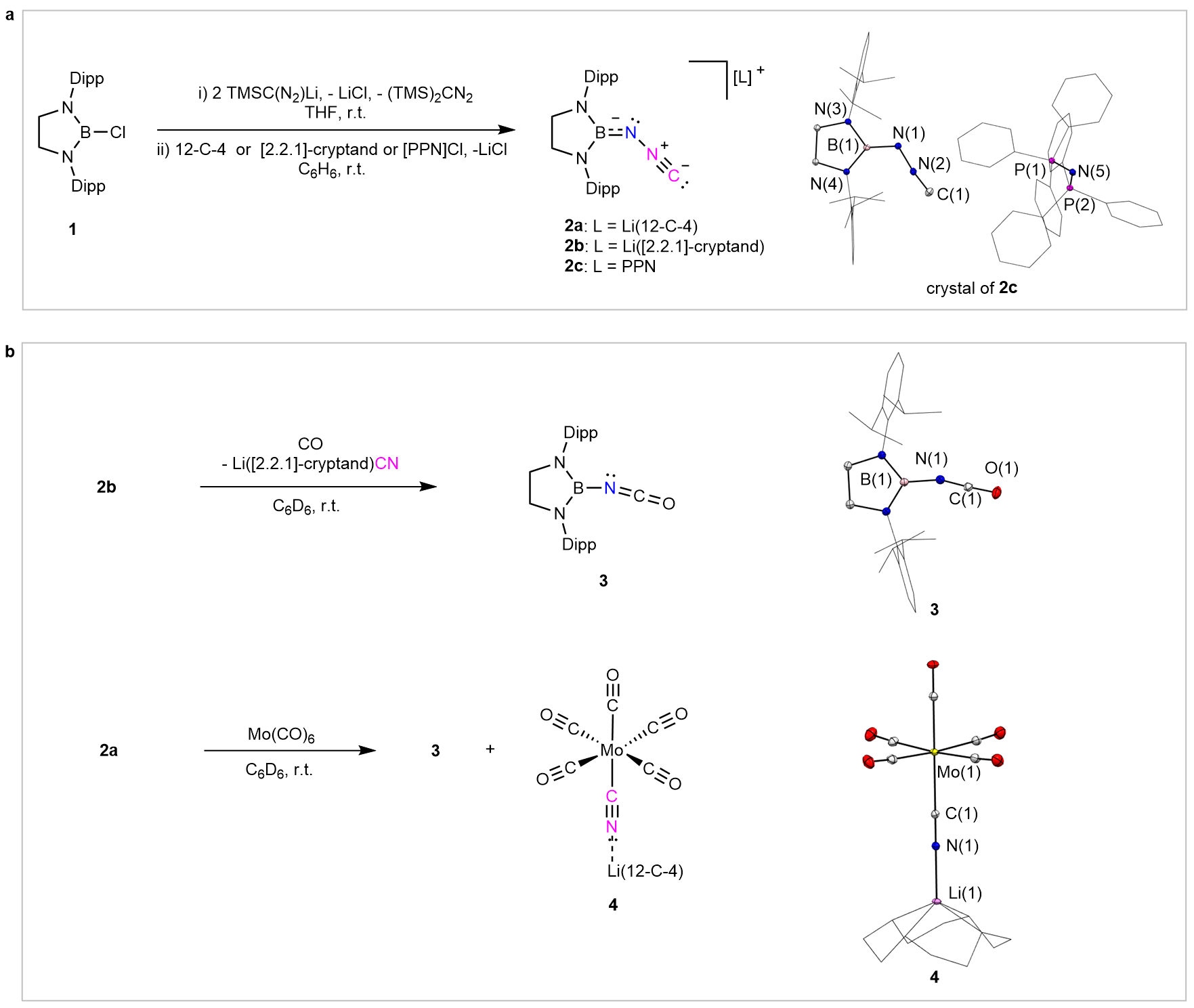
Figure 2: Synthesis of isodiazomethyl anion and its ligand exchange reaction.
Remarkably, the team designed a straightforward and efficient synthetic pathway. Starting from a chlorodiazaborolidine 1 and TMSC(N2)Li as the key building blocks (Fig. 2a), the reaction proceeds through a sequence of salt metathesis followed by desilylation steps, ultimately enabling the successful synthesis of the isodiazomethyl anion.
X-ray crystallographic analysis of 2c reveals that the NNC fragment of the isodiazomethyl anion adopts a nearly linear geometry (Fig. 2a). The anion exhibits distinctive reactivity, undergoing a concerted CN⁻/CO exchange at the nitrogen atom to give an isocyanate (Fig. 2b), as well as easy metathesis reactions with C=O, C=S, and C=N bonds (Fig. 3). Theoretical calculations indicate that the isodiazomethyl anion can be regarded as an adduct of a boryl-nitrene and a cyanide anion, formally acting as a nitrene transfer reagent and thus accounting for its unique and versatile reactivity.
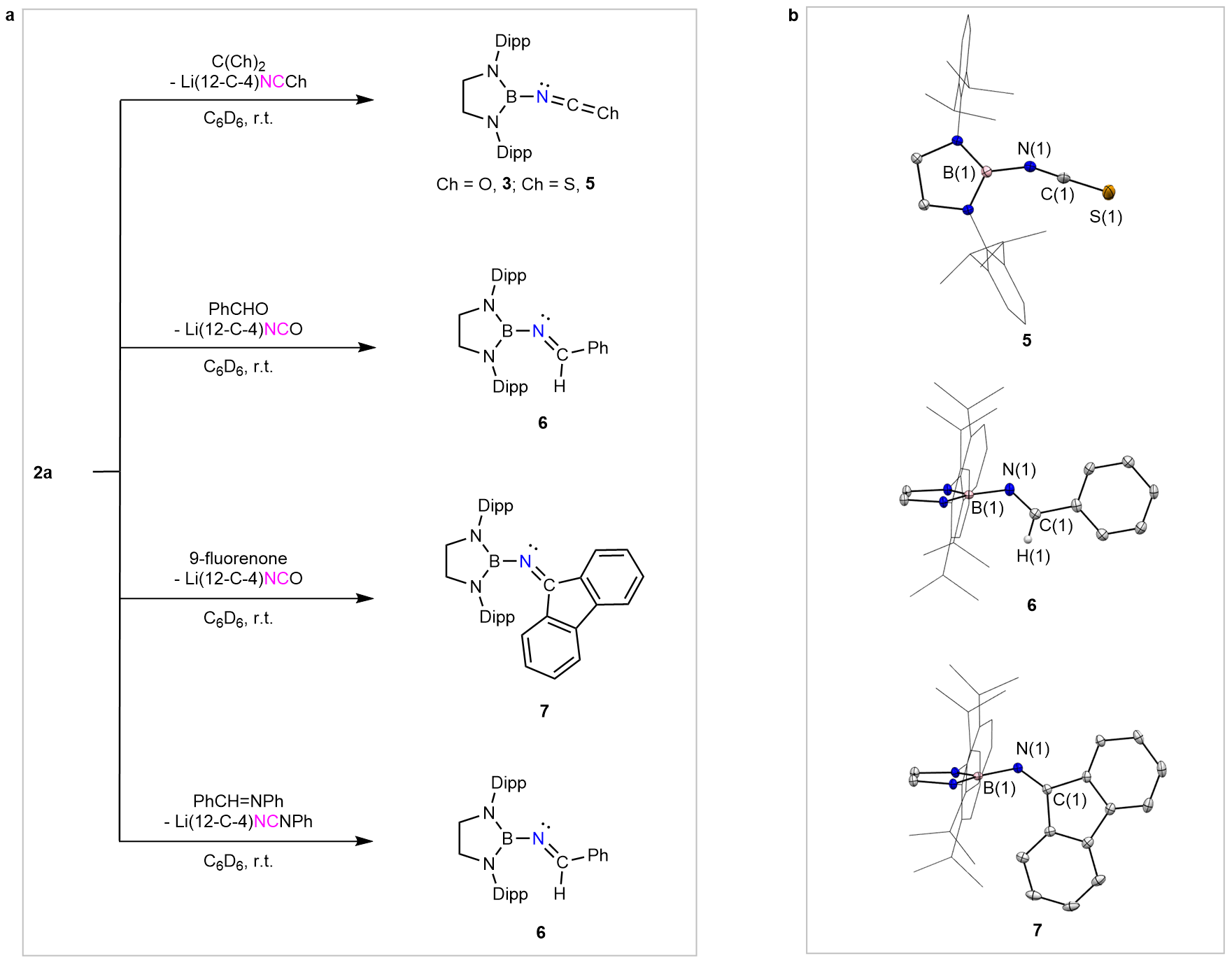
Figure 3: Metathesis reactions of isodiazomethyl anion.
This study provides an important foundation for the design of novel carbon-nitrogen functional groups and their applications in synthetic chemistry. The team is now exploring the reactivity of these compounds further, with a focus on creating previously uncharted functional groups.
The LIU research group has long been dedicated to fundamental studies in main-group chemistry. Based at Southern University of Science and Technology, the group has produced a series of influential publications in journals including Science, Nature, Nature Chemistry, Nature Synthesis, Chem, Nature Communications, CCS Chemistry, Journal of the American Chemical Society, Angewandte Chemie International Edition, and Accounts of Chemical Research. Many of these works have been highlighted by journals and platforms such as Science, Nature Synthesis, Chem, ChemistryViews, and ChemistryWorld as Editors’ Choice, Highlights, or Previews.
SUSTech is the sole affiliated institution for this work. Associate Professor Liu Leo LIU and Research Assistant Professor Jiancheng LI serve as co-corresponding authors, with Hongyu WANG, a PhD student from the 2022 cohort, as the first author.
Paper Link: https://www.nature.com/articles/s44160-025-00981-7
Proofread ByNoah Crockett, Junxi KE
Photo ByYan QIU