In chemical reactions, quantum interference phenomena are ubiquitous. It is challenging to understand the root cause of these interferences accurately. That is because of their complex interference. It is difficult to distinguish the characteristics of these interference patterns through experimentation.
On May 15, College of Science Dean Yang Xueming led his research teams at Southern University of Science and Technology and the Dalian Institute of Chemical Physics (Chinese Academy of Sciences) to publish a ground-breaking paper in the high-impact academic journal Science (IF = 37.2).
Their paper, titled “Quantum interference in H + HD → H2 + D between direct abstraction and roaming insertion pathways,” showed that in the simplest chemical reactions, there are still mechanisms to uncover. It proved that there is quantum interference and proving nature does play dice, as initially derided by Albert Einstein.
The most straightforward reaction is a hydrogen reaction, H + H2, and its isotopes. Since the reaction only involves three electrons, it is easier to calculate the interaction form in different configurations of the three atoms. That means it is easier to simulate and then realize the reaction process at the molecular level so that researchers can understand the chemical process at the micro-level.
The research teams found that at a specific scattering angle, the volume of H2 produced would oscillate at a specific rate relative to the collision energy. They applied improved technology to increase higher collision energy, thereby further developing quantum reaction scattering theory. In the process, the teams also developed an innovative method for analyzing chemical reactions through the topology.
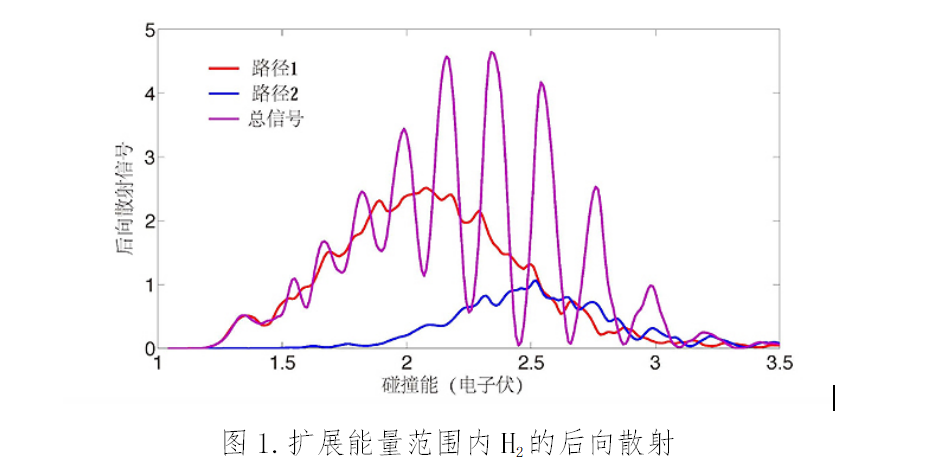
Topological analysis shows that the fluctuation in backscattering comes down to the interference from two chemical reaction paths, whose phase changes demonstrate an uptrend and downtrend respectively as the collision energy fluctuates.
Classical trajectory calculations suggest that one of the paths follows a direct reaction process. At the same time, the other shows a roaming mechanism-based reaction. As the two paths go around the H+HD reaction in opposite directions, the interference pattern shall be figured out through non-adiabatic coupling potential energy surface. That means it precisely mirrors the geometric phase effect.
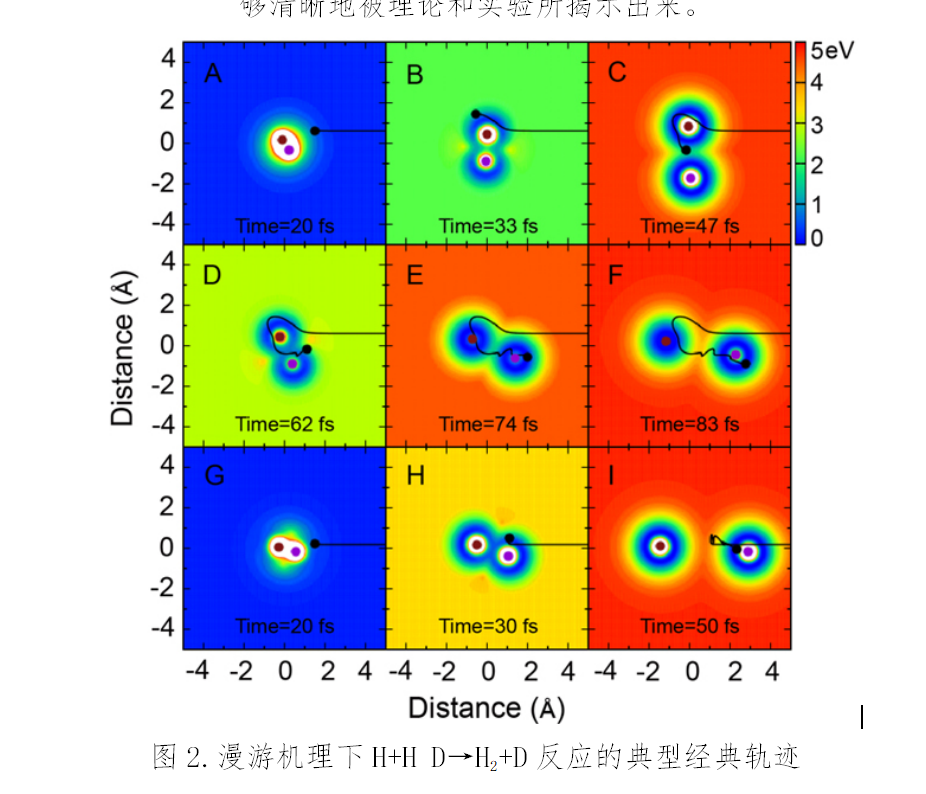
The discovery exposes the quantum fabric in collision-driven atom-molecule reactions. Further, it confirms the intricacy of chemical reaction paths.
Professors Xueming Yang, Chunlei Xiao, Zhigang Sun, and Dong H. Zhang are the corresponding authors of the paper. Drs. Yurun Xie and Hailin Zhao are the co-first authors of the paper.
This work was supported by the National Natural Science Foundation of China, the Chinese Academy of Sciences, and the Ministry of Science and Technology
Paper link: https://science.sciencemag.org/content/368/6492/767.full
Proofread ByYingying XIA
Photo ByDepartment of Chemistry, Yan QIU