Catalytic chemistry has been the source of considerable breakthroughs. New findings by chemists at Southern University of Science and Technology (SUSTech) has seen a new direction that could change commercial catalytic chemistry for years.
On May 18, Professor Xin-yuan Liu (Chemistry) has led his research group to publish a breakthrough paper. It was published in the high-impact academic journal, Nature Catalysis, under the title of “Cu-catalysed intramolecular radical enantioconvergent tertiary β-C(sp3)–H amination of racemic ketones.”
Professor Liu’s research group has been long focusing on developing Cu-chiral counteranion/ligand catalyzed asymmetric reactions involving radical intermediates. Ever since 2016, they have realized a series of asymmetric radical transformations, including alkene difunctionalization and cross-coupling using terminal alkynes and various alkyl halides as substrates.
The direct enantioselective functionalization of C–H bonds has been recognized as the optimal approach to construct carbon-centered enantioenriched molecules. This is because of its inherent economic and environmentally benign nature. However, the direct enantioconvergent transformations of racemic tertiary C(sp3)–H bonds have been rarely reported.
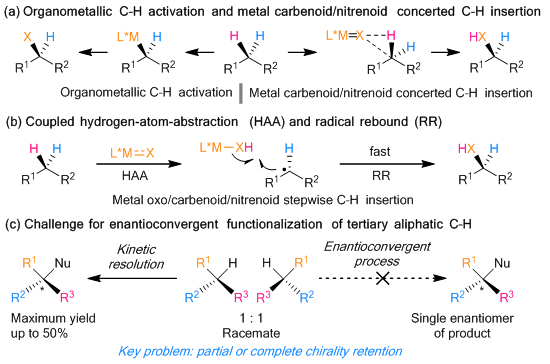
Figure 1. Transition metal-mediated asymmetric functionalization of C–H bonds
However, from the viewpoint of atom- and step-economy, direct oxidative coupling of universal C–H bonds bypasses the need to pre-functionalize starting materials. It makes it an ideal synthetic approach. The research group has just realized an asymmetric intramolecular amination of benzylic/allylic secondary C(sp3)–H bonds, using N-hydroxyphthalimide (NHPI) as a hydrogen shuttle.
Motivated by this success, they turned their attention to the more challenging asymmetric functionalization of racemic tertiary C(sp3)−H bonds, which remained unsolved for a long time. Inspired by the classic Hofmann-Löffler-Freytag reaction, they finally realized a highly enantioconvergent amination of tertiary β-C(sp3)–H of racemic ketones with copper/chiral phosphoric acid dual catalysis.
An essential feature of this work is that the transition metal is not directly involved in the formation of the tertiary carbon radical. It means that the rapid radical-rebound process associated with common transition metal-catalyzed stepwise nitrene/oxene C–H insertion is avoided. There is also an avoidance of the resulting chirality retention issues. The paper highlights the significant potential for the current strategy of developing enantioconvergent functionalization of tertiary C(sp3)–H bond.
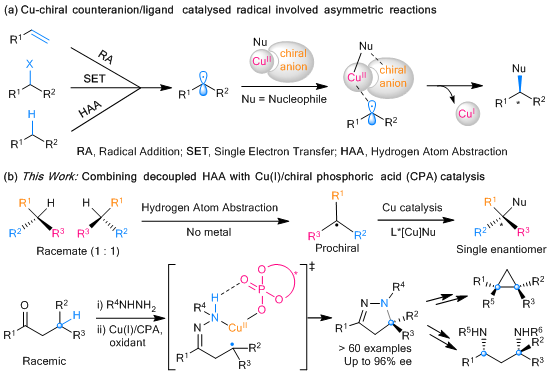
Figure 2. Cu-chiral counteranion/ligand catalyzed radical asymmetric chemistry
The co-first authors are SUSTech research scholars Chang-jiang Yang, Chi Zhang, and research assistant professor Qiang-shuai Gu. Additional contributions came from postgraduates Jia-heng Fang and Yu Tian, and undergraduate Yan Sun (’20, Chemistry). Professor Xin-yuan Liu is the sole corresponding author, and SUSTech is the sole communication unit.
Financial support from the National Natural Science Foundation of China, Shenzhen Special Funds, Shenzhen Nobel Prize Scientists Laboratory Project, and ‘Fundamental Research Funds for the Central Universities’ is gratefully acknowledged.
Paper link: https://www.nature.com/articles/s41929-020-0460-y
Proofread ByYingying XIA
Photo ByDepartment of Chemistry, Yan QIU