The development of molecular recognition technology that mimics nature has been progressed following a series of studies by researchers at Southern University of Science and Technology (SUSTech).
The research team of Professor Wei JIANG (Chemistry) has made significant developments in biomimetic molecular recognition and its applications. Their research outputs were published in high-impact academic journals. The team was also invited to publish highlight and review articles on their outcomes.
Molecular recognition is central to all biological processes and supramolecular chemistry. It may provide the final solution for some problems in biomedical, materials, and environmental science. Macrocyclic hosts are the primary tools used in supramolecular chemistry as they can recognize small molecules through non-covalent interactions. However, known macrocyclic hosts are often not comparable to bioreceptors in terms of recognition ability and selective, adaptability. It may be due to the lack of functional groups inside the deep cavity, which is a common feature of bioreceptors.
Since 2013, the research group has developed a series of naphthol-based macrocycle hosts with a biomimetic cavity feature. They have solved some generally accepted challenges in supramolecular chemistry. Their macrocyclic hosts demonstrate unique applications in sensing, materials, machines, and assembly
The first paper was published in CCS Chemistry, a flagship academic journal of the Chinese Chemical Society. The paper was titled “Enantioselective Recognition of Neutral Molecules in Water by a Pair of Chiral Biomimetic Macrocyclic Receptors.”
Enantioselective recognition in water is the basis of life. Natural receptors are made up of chiral building blocks, creating chiral binding pockets highly selective towards chiral substrates. However, the enantioselective recognition of molecules in water is exceptionally challenging with synthetic receptors.
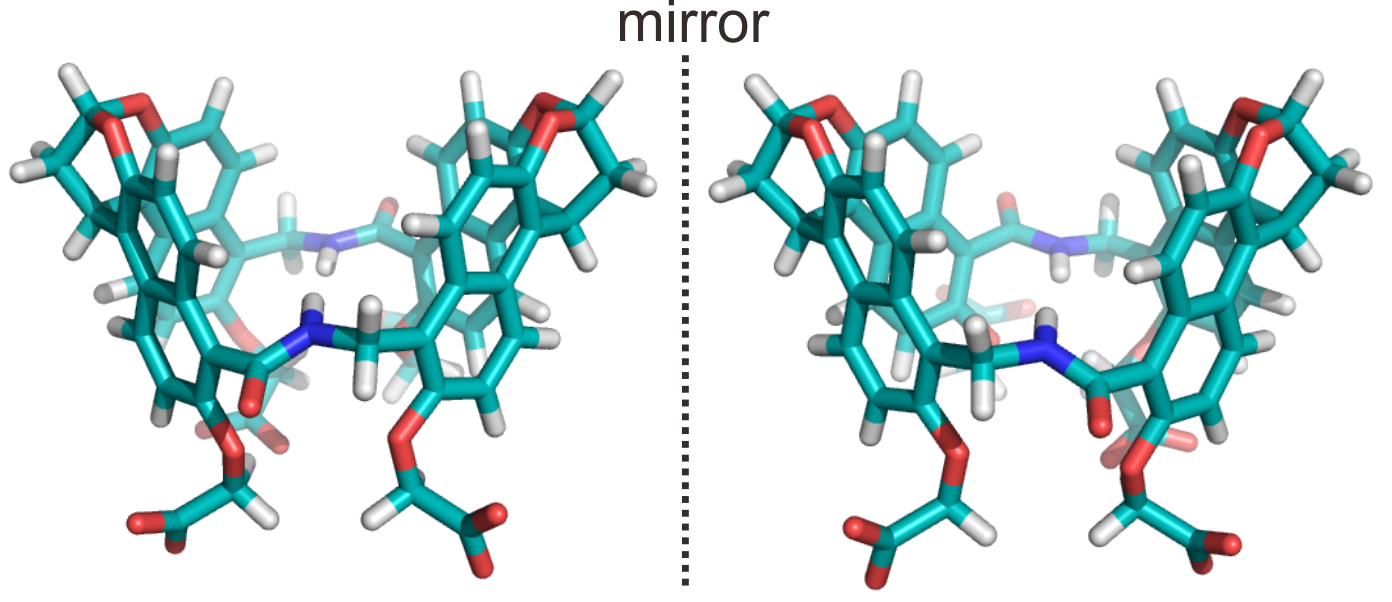
Figure 1. Structural models of the chiral naphthotubes
In their paper, the researchers synthesized the first enantio-resolved pair of biomimetic receptors by mimicking the cavity feature of bioreceptors (Figure 1). The chiral naphthotubes can discriminate against the enantiomers of neutral chiral molecules in water. These chiral naphthotubes are both fluorescent and CD-active. They demonstrated that the chiral naphthotubes could detect nonchromophoric achiral molecules in water under CD spectroscopy. The use of fluorescence spectroscopy aided in the determination of the enantiomeric excess values of chiral molecules.
The paper published in Nature Communications (IF = 11.878) was titled, “A supramolecular system that strictly follows the binding mechanism of conformational selection.” The conformational change in biological receptors is fundamental to many biological functions. Macrocyclic hosts are structurally simple and can be used as simplified models to reveal detailed mechanisms of conformational changes in molecular recognition.
The group reported a macrocyclic receptor that strictly follows the binding mechanism of conformational selection. They also provided an in-depth analysis of the kinetic and thermodynamic aspects of the system.

Figure 2. The binding mechanism of conformational selection
The paper titled, “Redox-Responsive Host-Guest Chemistry of a Flexible Cage with Naphthalene Walls” was published in the high-impact academic journal, Journal of the American Chemical Society (JACS) (IF = 14.695). The group created a flexible naphthol-based cage that adopts a self-inclusion conformation. It can bind singly charged organic cations. Their research proved that flexibility is not necessarily the enemy of high-affinity binding.
As part of their research, the group found that the cage can be reversibly oxidized to its radical cation, which can be applied for the controlled release from the cage. It paves the way for new applications in the redox-controlled guest release or stimuli-responsive materials.
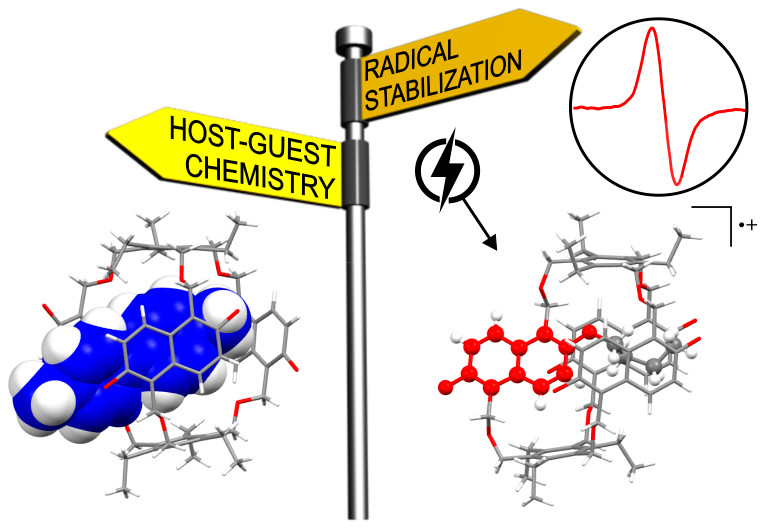
Figure 3. The redox-responsive naphthocage
The research group wrote a review article in the high-impact academic journal, Accounts of Chemical Research (IF = 21.661). The paper was published, “Naphthotubes: Macrocyclic Hosts with a Biomimetic Cavity Feature.” It sought to summarize their efforts on naphthotubes in the last five years.
They discussed the modular synthesis and molecular recognition of napthtotubes, while also reviewing its potential applications. The team provided an outlook on the design of novel macrocyclic hosts and their applications.
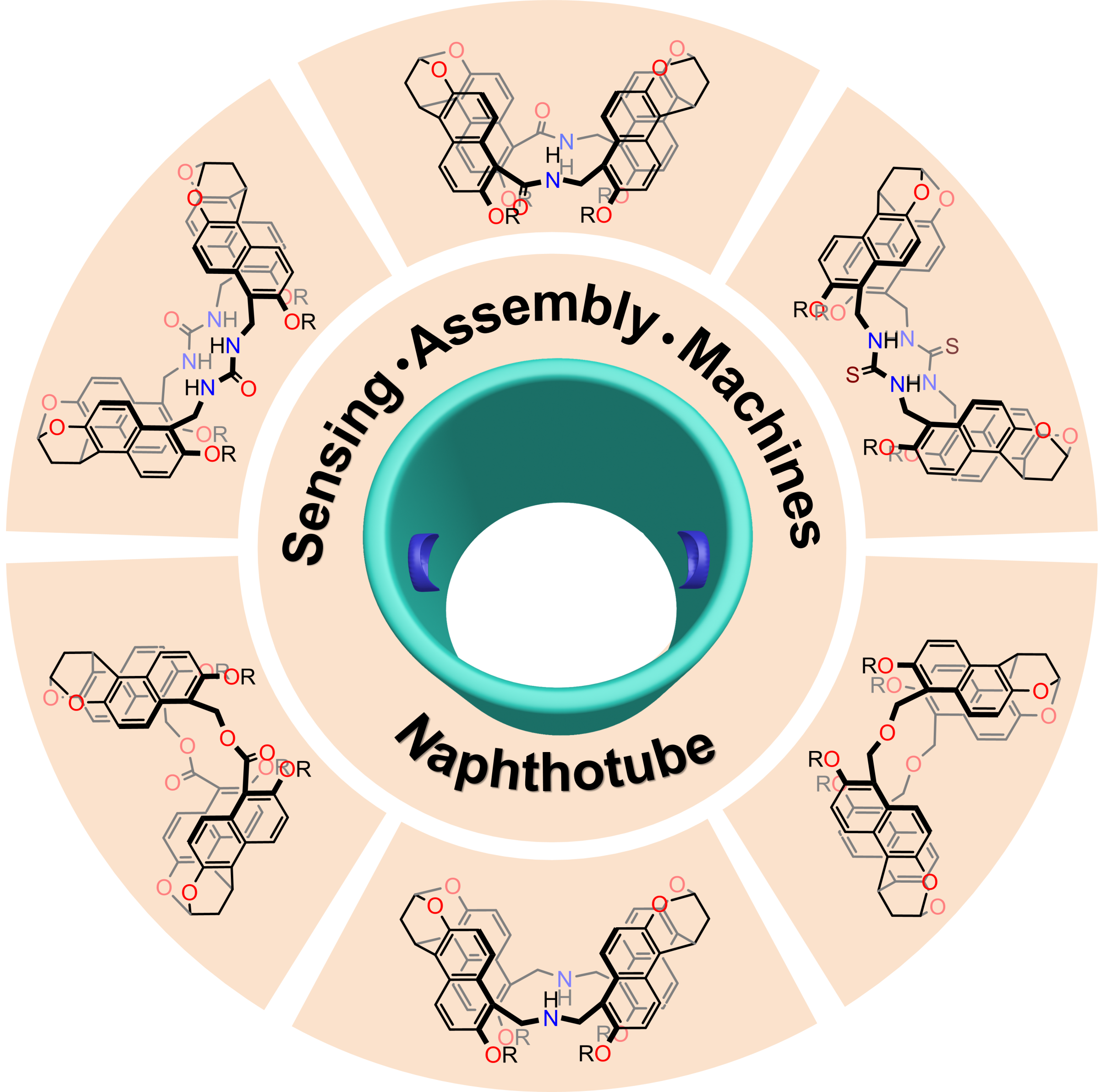
Figure 4. Chemical structures of naphthotubes (not all)
A different review paper was published in Chemical Society Reviews (IF = 40.443). The paper, titled “Conformationally Adaptive Macrocycles with Flipping Aromatic Slidewalls.” The review paper examined the conformational properties and characteristics of macrocycles with aromatic sidewalls. They demonstrated that the macrocycles are unique in terms of their chiral sensing, stimuli-responsive self-assembly, and molecular switches. The research group focused on zorbarenes, oxatubarenesss, and naphthocages that they had developed.
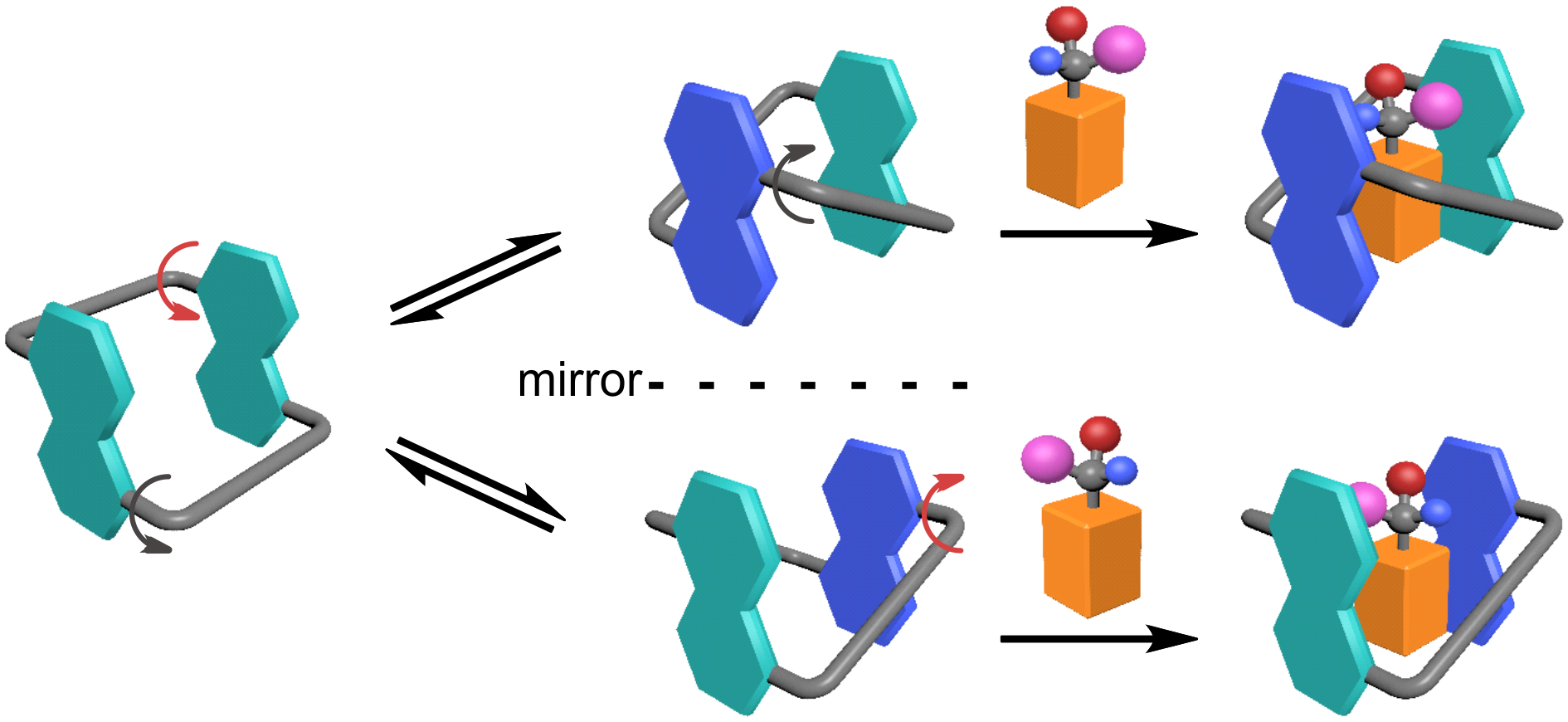
Figure 5. Conformationally adaptive macrocycles with aromatic sidewalls
The group published a highlight article in Angewandte Chemie International Edition (Angew. Chem. Int. Ed.) (IF = 12.257). Their highlight article was titled, “Prismarene: An Emerging Naphthol-Based Macrocyclic Arene.” It sought to comment and provide an outlook on a recently reported novel macrocyclic host.

Figure 6. Optimized structure of prism[n]arene
The co-first authors of these papers are doctoral candidates of joint programs Zhao Chen and Fei Jia, postdoctoral researchers Hongxin Chai, senior research scientist Xiaoping Wang, and research assistant professor Liu-Pan Yang. Professor Jiang Wei is the sole or co-corresponding author for all the papers, with SUSTech as the sole or co-communication unit.
Financial support from the National Natural Science Foundation of China, Guangdong Special Support Plan, Shenzhen Science and Technology Innovation Committee,Shenzhen “Pengcheng Scholar,” Guangdong Provincial Key Laboratory of Catalysis and Shenzhen Nobel Prize Scientists Laboratory Project is gratefully acknowledged. They also acknowledge the Center for Computational Science and Engineering at SUSTech for theoretical calculation support, and the SUSTech Core Research Facilities for technical support.
Proofread ByChris Edwards, Yingying XIA
Photo ByDepartment of Chemistry, Yan QIU