Research by Southern University of Science and Technology (SUSTech) presents the 3.7-Å-resolution structure of the Varicella-zoster virus (VZV) A-capsid, which defines the molecular determinants underpinning the assembly of this complicated viral machinery. Their observations highlight the divergence of different herpesviruses and provide an essential basis for developing antiviral drugs.
The research team led by Professor Peiyi Wang (Biology, Cryo-EM Center) just published a new paper entitled “Cryo-EM structure of the varicella-zoster virus A-capsid.” in the high-impact academic journal, Nature Communications (IF = 13.61). They used cryo-electron microscopy to analyze the delicate three-dimensional structure of the VZV capsid.
VZV widely exists in nature. After infection, there will be groups of blisters on the surface of the skin. In severe cases, necrotic ulcers occur. Diseases such as pneumonia, encephalitis, and some immune system diseases can occur. About thirty thousand people, mostly children, die every year from various VZV-related diseases. VZV adopts a unique mechanism of maintaining long-term latency in neurons of the spinal cord. Under specific conditions, they can be reactivated and resume proliferation. It is thus difficult to completely eradicate the virus in infected patients who will carry the virus for life.
VZV is an enveloped double-stranded DNA virus with a diameter of 150-200nm. The virion comprises four layers, consisting of the surface capsule, the intermediate protein layer, the capsid, and the DNA genome. The VZV capsid is very complicated, consisting of thousands of protein subunits, a formidable challenge in structural biology. The capsid is very important for protecting the viral genome. It plays vital roles in virus assembly, maturation, and infection.
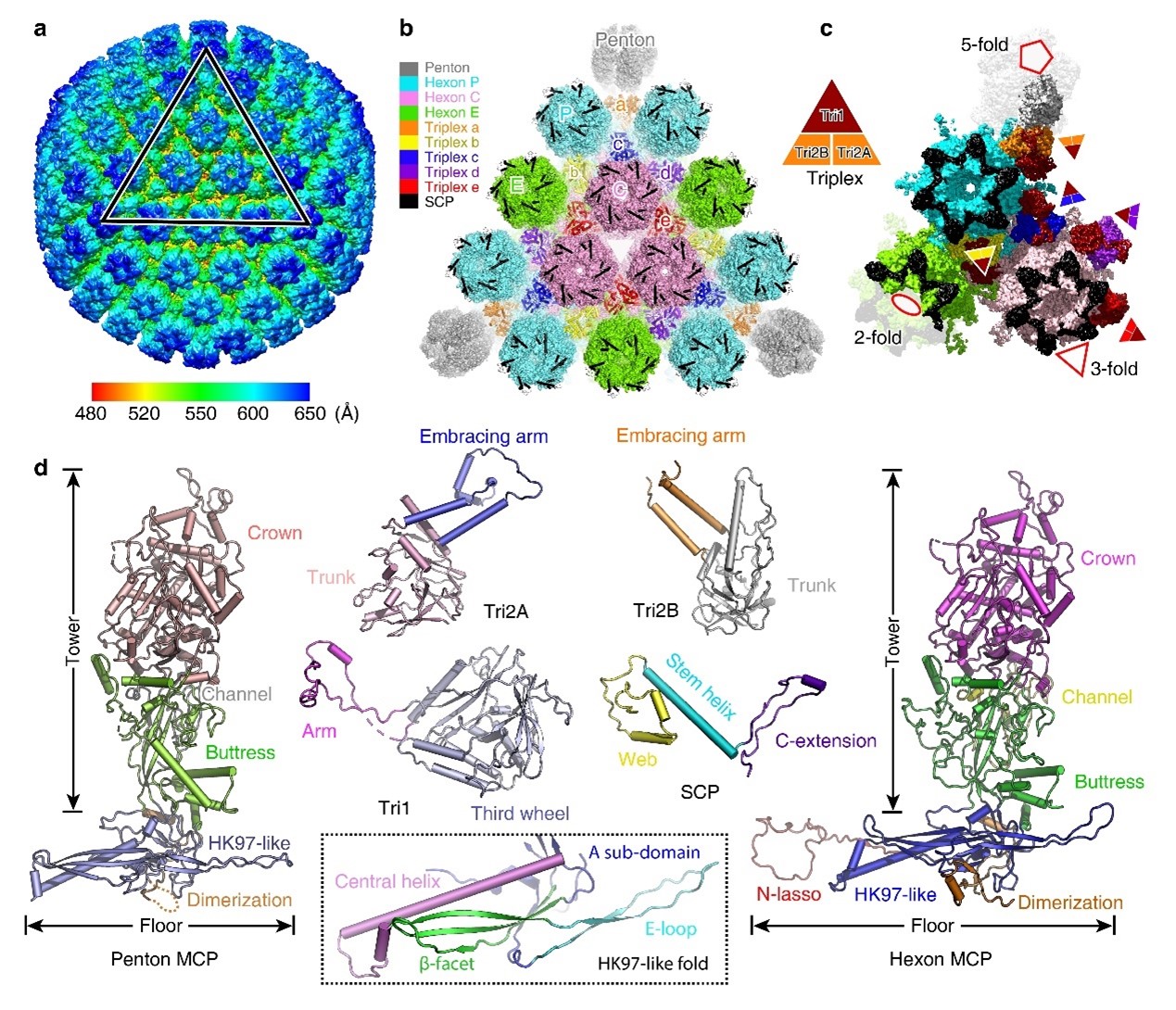
Figure 1 The overall structure of VZV type A capsid
The VZV capsid mainly includes four protein components: the major capsid protein (MCP), the small capsid protein (SCP), and the triplex protein 1 (Tri1) and 2 (Tri2), which are arranged in icosahedral symmetry. The MCP arranges into pentons at the icosahedral vertexes or hexons within the facet. Compared to other known human herpesvirus structures, the interface between penton and hexon capsomers is more flexible with fewer interaction networks between MCP subunits. This phenomenon might lead to the lower stability of the VZV capsid than other human herpesviruses. It could be related to lower internal pressure rendered by its relatively smaller genome inside the capsid.
The research team compared the features of the three different herpesvirus subfamilies. They found that the C-terminal portion of SCP and the internal insertion loop of the Tri1 subunit display evident diversity among these viral groups. These unique structural features indicate potential targets for developing antiviral drugs against specific viruses. Previous studies have reported that a pyrazole derivative 35B2 can bind to the VZV capsid protein to block viral capsid assembly. By analyzing this structure and escape mutations, the authors identified a “pocket” under the MCP base that could be the potential binding site of this compound. This “pocket” also implies a potential target for antiviral drug design.
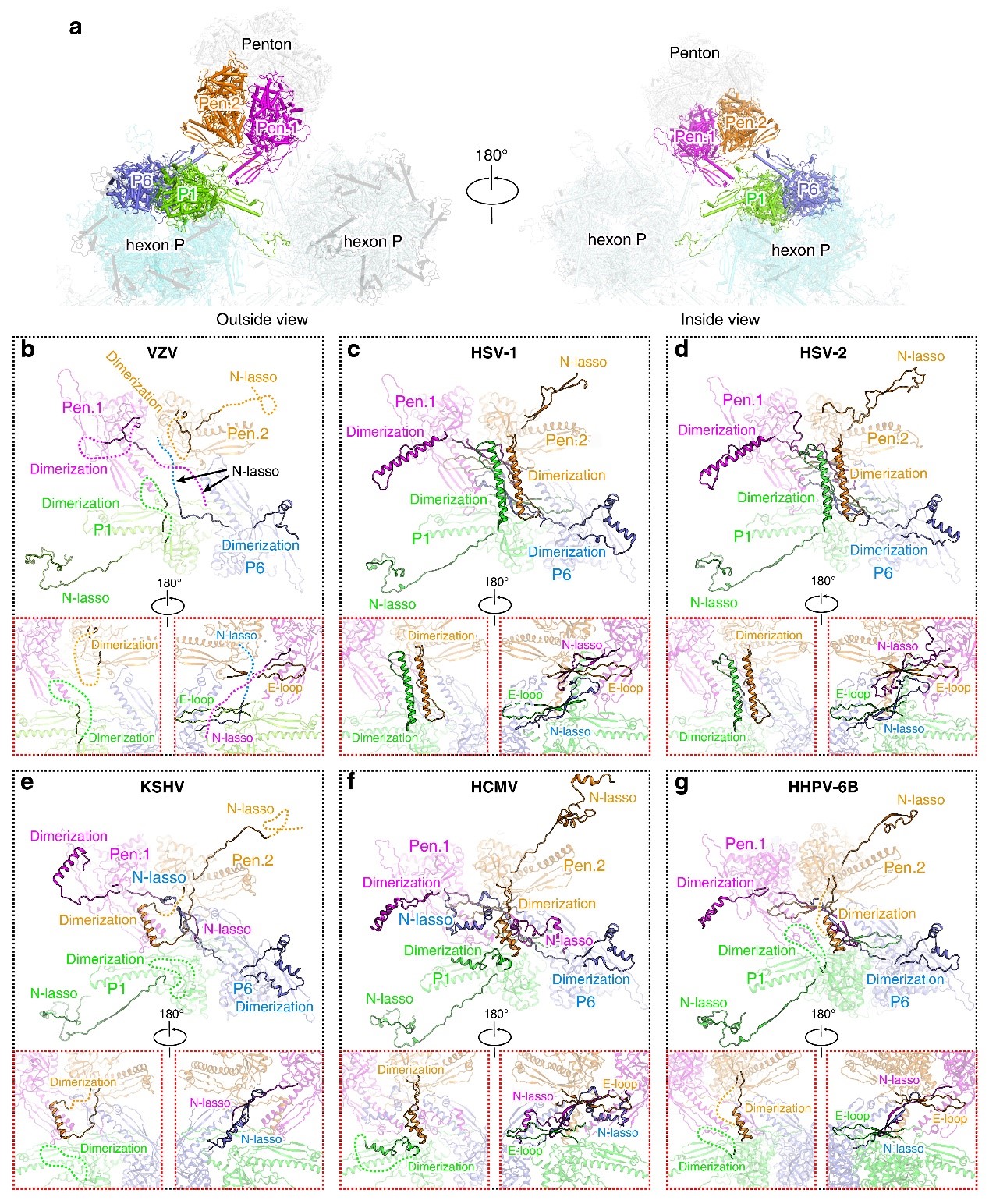
Figure 2 Comparing the interaction interface of different herpes viruses capsid assembly
Dr. Peiyi Wang, Professor Jianxun Qi (Institute of Microbiology (CAS)), Professor Minhua Luo Wuhan Institute of Virology (CAS), and Professor Xiaoming Yang (Chinese National Pharmaceutical Group (Sinopharm)) co-supervised the study. Additional contributions came from the Shanxi Academy of Advanced Research and Innovation, the University of the Chinese Academy of Sciences (UCAS), and Changchun Keygen Biological Products.
The Cryo-EM data was collected at the SUSTech Cryo-EM Center. This work was supported by the Strategic Priority Research Program of CAS, National Natural Science Foundation of China (NSFC), and the Technological Innovation Project of Shanxi Transformation and Comprehensive Reform Demonstration Zone. P.W. is supported by a startup grant from the Southern University of Science and Technology.
Proofread ByYingying XIA
Photo ByDepartment of Biology, Cryo-EM Center, Yan QIU