Recently, the team of Associate Professor Lele Duan from the Department of Chemistry of the Southern University of Science and Technology (SUSTech) reported a rapid and efficient method of preparing graphdiyne (GDY)-supported transition metal single-atom and sub-nanocluster catalysts and explored their application in the field of energy catalysis. The results were published in the internationally renowned chemistry journals Angew. Chem. Int. Ed. and Proc. Natl. Acad. Sci. USA.
The catalytic activity of transition metal single-atom and sub-nanocluster catalysts is strongly dependent on their supporting materials. Usually, materials containing defects or doped with heteroatoms prepared by high-temperature methods have complex metal coordination environments, which complicates the study of catalyst structure-activity relationship and the reaction mechanism. Therefore, the preparation of a single-atom and cluster catalysts with a clear coordination environment is of great significance to understanding the catalytic mechanism and structure-activity relationship. In view of this, Dr. Duan’s group used GDY with a clear and ordered structure as the support. They designed a method of acetylene bond induction-site trapping to prepare a series of GDY-supported metal single-atom and sub-nanocluster catalysts (Figure 1), and studied their catalytic activity and reaction mechanism in the electrochemical reduction of CO2, CO, and N2.
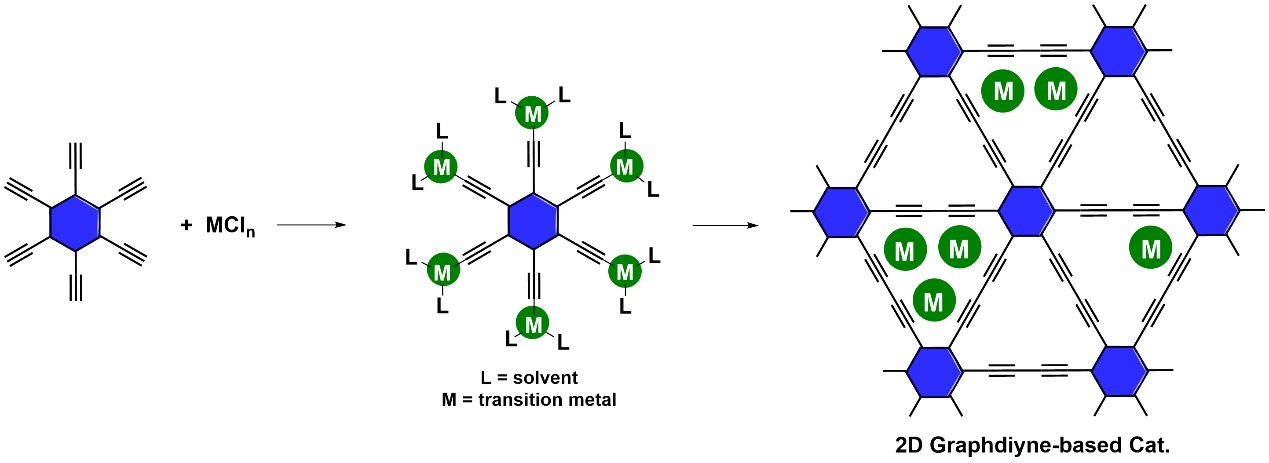
Figure 1. Schematic illustration for the synthesis of M/GDY catalysts
Electrocatalytic CO2/CO reduction can convert CO2 or CO in industrial production into higher value-added hydrocarbons. Cu-based catalysts are often used for the electrochemical reduction of CO2/CO. However, little progress has been made in regulating the size of Cu nanoclusters at the atomic level. The team used the newly developed synthetic method to successfully prepare three GDY supported copper catalysts with different sizes, which are 1–1.5 nm nanoclusters (NCs, Cu45.2/GDY), 0.5–1 nm sub-nanoclusters (SCs, Cu6.0/GDY), and single-atom catalysts (SAs, Cu1.5/GDY). The size effect of copper catalysts on the reduction of CO2/CO at the atomic scale was studied (Figures 2 and 3). At the same time, the confinement effect formed by the porous structure of GDY and a large number of acetylene bonds greatly increased the interaction between the metal and the substrate and ensured the stability of the catalyst. 【Angew. Chem. Int. Ed. 2021, 60, 466-472.】
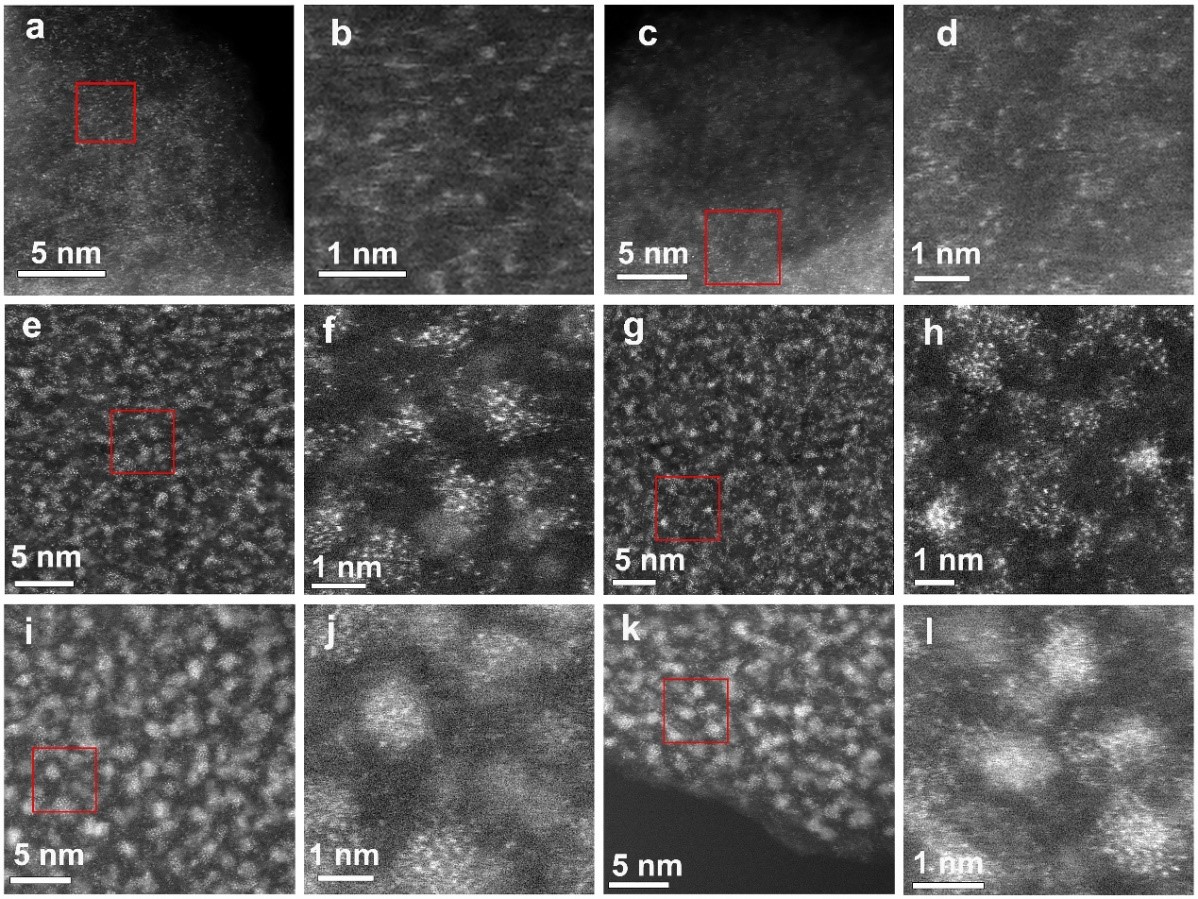
Figure 2. HAADF-STEM images of Cu/GDY with different sizes
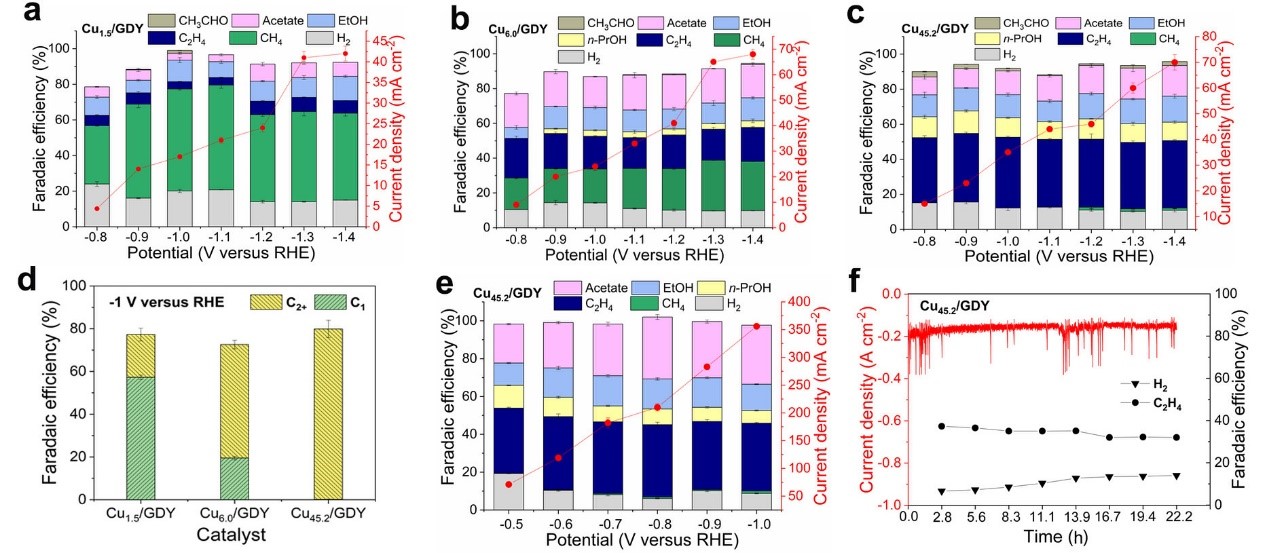
Figure 3. The CO2 electroreduction performance of three catalysts
Using renewable electricity to synthesis ammonia from nitrogen paves a sustainable route to making value-added chemicals but requires further advances in electrocatalyst development and device integration. By engineering both electrocatalyst and electrolyzer to simultaneously regulate chemical kinetics and thermodynamic driving forces of the electrocatalytic nitrogen reduction reaction (ENRR), stereo-confinement induced densely populated metal single-atoms (Rh, Ru, Co) on GDY matrix (formulated as M SA/GDY) achieved an increased ENRR activity at the pressurized reaction system.
Remarkably, under the pressurized situation, the originally highly active hydrogen evolution reaction of M SA/GDY was effectively suppressed while the desired ENRR activity was strongly amplificated. As a result, the pressurized ENRR activity of Rh SA/GDY at 55 atm exhibited a record-high NH3 formation rate of 74.15 μg h-1 cm-2, a FE of 20.36%, and an NH3 partial current of 0.35 mA cm-2 at -0.20 V versus reversible hydrogen electrode, which respectively displayed 7.3-, 4.9- and 9.2-folds enhancements compared with those obtained under ambient conditions.
Furthermore, a time-independent ammonia yield rate using purified 15N2 confirmed the concrete ammonia electroproduction. In this work, the proposed strategy and findings provided a valuable electrocatalysis scheme with the capability to cooperate efficient catalysts and a pressurized reaction system, which is a step forward for the electrochemical ammonia production.【Proc. Natl. Acad. Sci. U.S.A. 2020, 117, 29462-29468.】
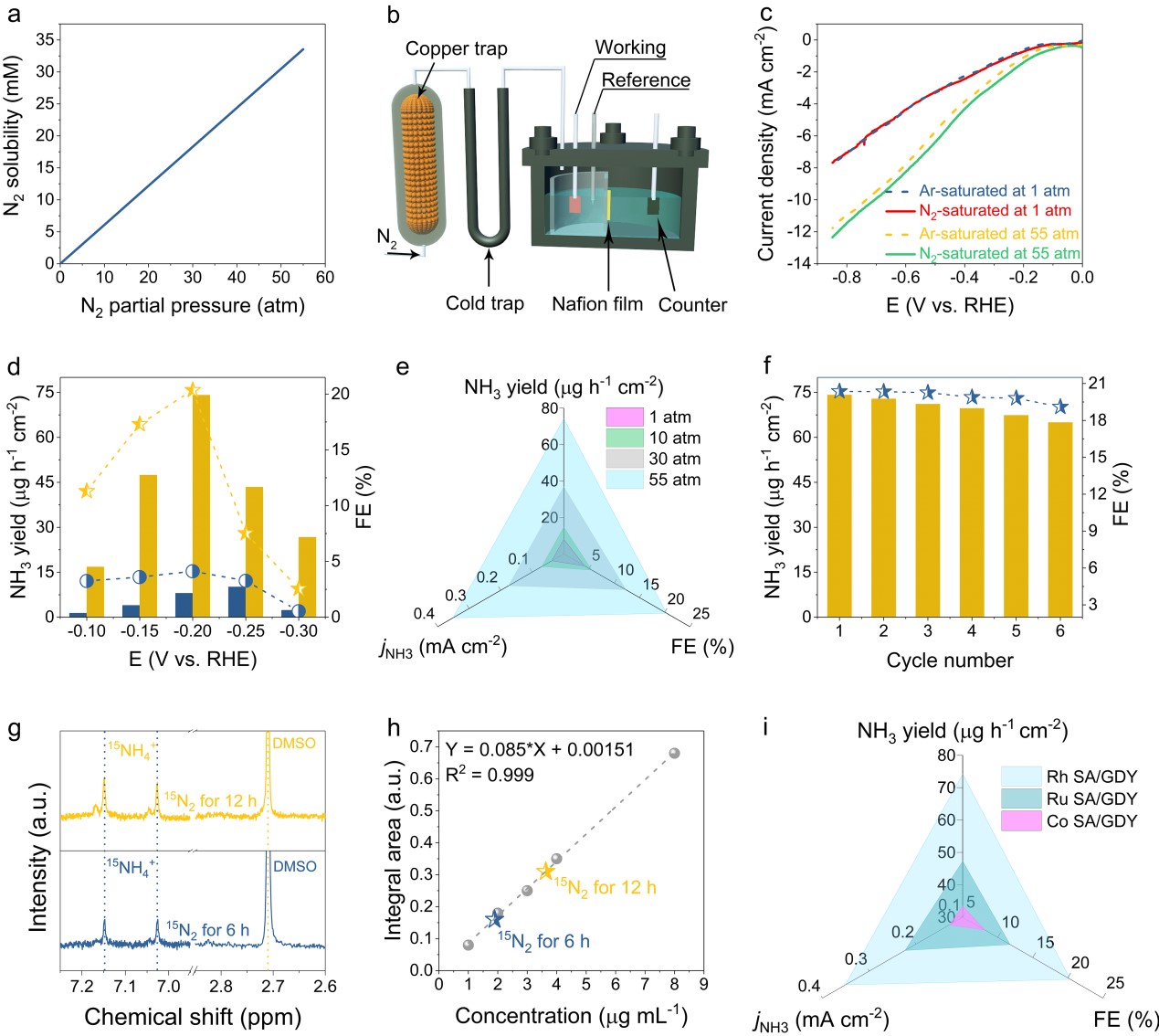
Figure 4. Pressurized ENRR measurements. (A) A plot of the solubility of N2 gas versus the partial pressure. (B) Schematic of the home-made pressurized ENRR setup. (C) Ar- and N2-saturated LSV curves of Rh SA/GDY under ambient and 55-atm conditions, respectively. (D) The NH3 yields and their corresponding FEs of Rh SA/GDY under ambient and 55-atm conditions, respectively. (E) Comparison of the NH3 yield, FE, and jNH3 for Rh SA/GDY versus the exerted N2 pressures. (F) Cycling stability results of Rh SA/GDY at −0.2 V. (G and H) 1H NMR spectra and corresponding 15NH4+ yield with the isotopic 15N2 for 6- and 12-h electroreduction. (I) Comparison of the optimum NH3 yield, FE, and jNH3 for the prepared Rh, Ru, and Co SA/GDY.
Weifeng Rong, a postdoctoral fellow, and Haiyuan Zou, a Ph.D. student from SUSTech are respectively the first authors of these two papers. Professor Ji from Guangzhou University provides the theoretical support. The work is supported by the National Natural Science Foundation of China, the Science and Technology Innovation Commission of Shenzhen Municipality, Shenzhen Clean Energy Research Institute, and Guangdong Provincial Key Laboratory of Energy Materials for Electric Power. The authors would like to acknowledge the contribution from the core research facilities of the SUSTech in material characterizations.
Paper links:
https://onlinelibrary.wiley.com/doi/abs/10.1002/anie.202011836
Proofread ByAdrian Cremin, Yingying XIA
Photo By