The structure of solid matter can be divided into crystalline state and amorphous state in general. The difference between them lies in the order of atomic arrangement. In the periodic table of elements, almost all the common crystalline structures of the elements have been found. Still, up to now, not all elements have been reported to present an amorphous state. Therefore, whether all elements have an elemental amorphous state has always been an important puzzle in physics. In addition, single-element amorphous materials are also of great significance for understanding the structure and nature of amorphous substances (once was one of the 125 important problems proposed by Science journals).
Chair Professor Jiaqing He’s research team from the Department of Physics at the Southern University of Science and Technology (SUSTech) and his colleagues recently made important progress in the first synthesis of single-element amorphous palladium nanoparticles and its application in hydrogen storage. Single-element amorphous palladium nanoparticles were discovered in the experiment course of Advanced Electron Microscopy, conducted by Prof. He in early 2016.
After many years of repeated argumentation with domestic and international colleagues, single-elemental amorphous palladium nanoparticles were confirmed. His research, entitled “Single-element amorphous palladium nanoparticles formed via phase separation,” was published in Nano Research, an international and interdisciplinary research journal that focuses on all aspects of nanoscience and nanotechnology.
Single-element amorphous materials are metastable, which is extremely difficult to obtain, and harsh conditions are generally needed in the preparation process. At present, the primary way to obtain single-element amorphous materials is to heat and melt them and then cool them quickly. In the solidification process, the cooling rate needs to exceed the crystallization rate (> 1012 K/s) so that the atomic arrangement can be disordered. Even so, only a few body-centered cubic metals (such as tantalum, vanadium, and tungsten) can be cooled to an amorphous state, but there are few reports on closest-packed metals.
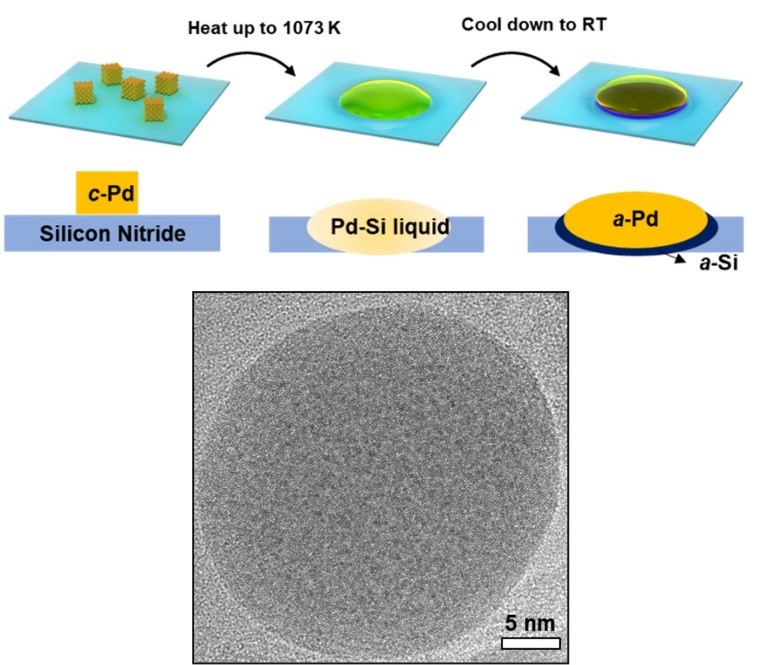
Figure 1 Schematic diagram of the synthetic route of single-element amorphous palladium nanoparticles (upper). Transmission electron microscope image of a typical single-element amorphous palladium nanoparticle (lower).
In this research work, Prof. He’s team and his collaborators found that single-element amorphous palladium can be formed by a phase separation mechanism, as shown in Figure 1. The researchers heated palladium on amorphous silicon nitride, forming a palladium-silicon eutectic melt. During the cooling process, the phase separation was produced by the preferred solidification of silicon on silicon nitride substrate, which leads to a large degree of undercooling of palladium. Because the substrate was amorphous, the arrangement of further solidified palladium atoms was templated as disordered. According to this mechanism, researchers could obtain up to hundreds of nanometers of amorphous palladium without too fast a cooling rate. The purity of amorphous palladium nanoparticles measured by atom probe tomography was 99.35 ± 0.23%, which exceeds the purity of commercial elemental palladium, as shown in Figure 2.
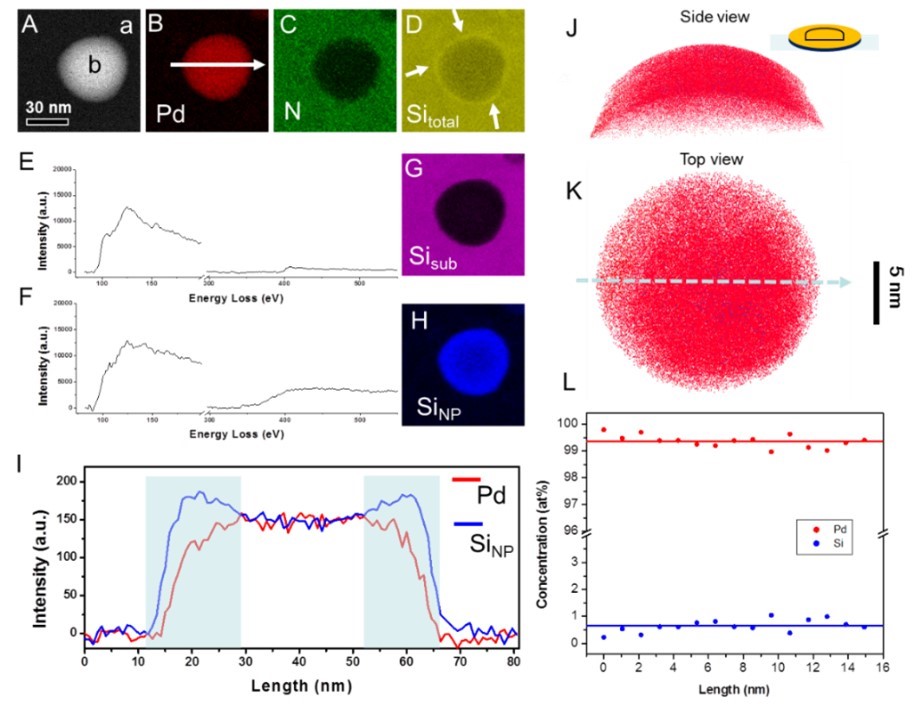
Figure 2 (A-D) element distributions; (E) and (F) are EELS spectra collected at positions (a) and (b) in figure (A), showing that silicon exists in different chemical states (silicon nitride and amorphous silicon); (G-H) Distribution of silicon in different chemical states; (I) Line profiles of the distributions of palladium and amorphous silicon; (J), (K) and (L) are a side and top view of the three-dimensional compositional distributions and one-dimensional compositional profiles of palladium (red dots) and silicon (blue dots) in the nanoparticle characterized by atom probe tomography.
On the other hand, palladium is a metal with strong interaction with hydrogen, which is often used for catalysis, hydrogen storage, and purification. Still, it also suffers from hydrogen embrittlement because hydrogen atoms enter the lattice and expand the lattices. The researchers tested the amorphous palladium in a hydrogen environment and found that although amorphous palladium absorbed less hydrogen than crystalline palladium, it hardly expanded. According to the simulations, the hydrogen atoms in amorphous palladium could selectively occupy the free space in solid and only occupy positions with ample space, so the lattice expansion was avoided, as shown in Figure 3.
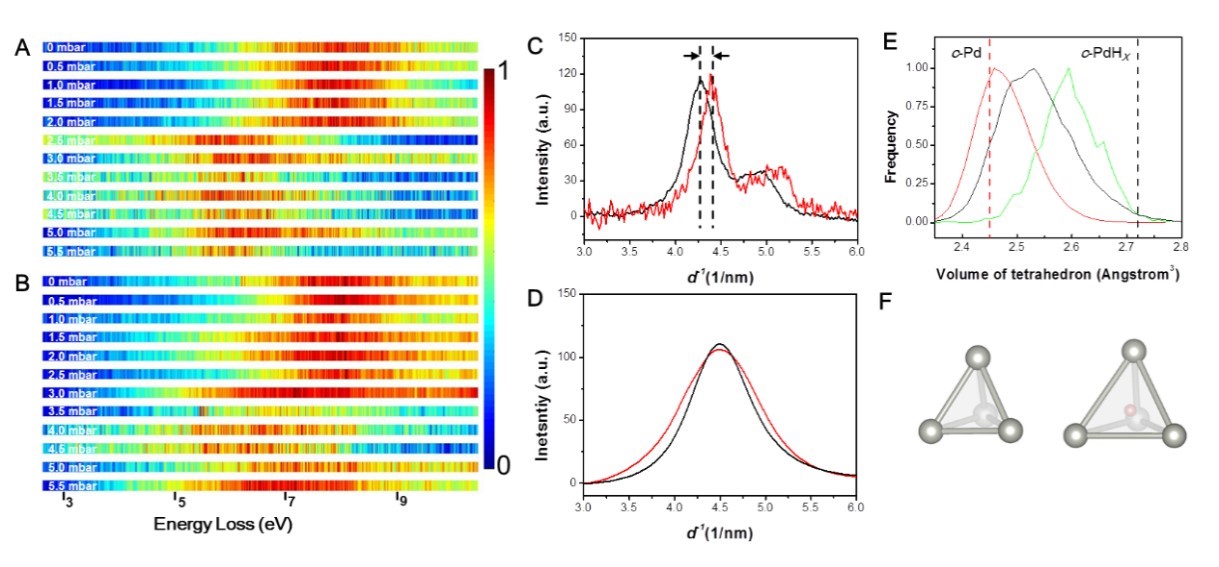
Figure 3. (A) and (B) show the low-loss EELS spectra of crystalline palladium and amorphous palladium as a function of hydrogen pressures, respectively; (C) and (D) are the changes in electron diffraction profiles of crystalline palladium and amorphous palladium before and after hydrogen absorption; (E) Volume change of tetrahedron formed by atoms of amorphous palladium before and after hydrogen absorption; (F) Two representative tetrahedral cages of amorphous palladium after hydrogen absorption according to molecular dynamics simulations (left: no hydrogen atoms inside, right: a hydrogen atom inside).
Palladium is a classical template material for crystallinity regulation for many noble metals. Amorphous palladium may induce the formation of a variety of amorphous noble metals, thus providing a new degree of freedom for their applications. This study also provides a novel insight into the synthesis of single-element amorphous materials under mild conditions.
Dr. Dongsheng He, an experimental teaching assistant of the Advanced Electron Microscopy, and Huang Yi, a joint doctoral student of SUSTech and the Harbin Institute of Technology (HIT), are the co-first authors of this paper. Chair Prof. Jiaqing He from SUSTech is the correspondent author. Collaborators include team members of Prof. Vinayak Dravid at Northwestern University, Prof. Li Huang of the Department of Physics, and Prof. Yang-Gang Wang of the Department of Chemistry at SUSTech.
This work was supported by the National Natural Science Foundation of China (NSFC), Guangdong Leading Talents Program, Guangdong Natural Science Foundation of China, Shenzhen Science and Technology Innovation Commission, and the SUSTech High-level Phase II Highlight Project. The Core Research Facilities and the Supercomputing Platform at SUSTech also provided strong support for this research.
Paper link: https://doi.org/10.1007/s12274-022-4173-1
To read all stories about SUSTech science, subscribe to the monthly SUSTech Newsletter.
Proofread ByAdrian Cremin, Yingying XIA
Photo By