Liquid organic hydrogen carriers (LOHCs) are small molecules with high hydrogen content. Common examples include low-molecular weight alcohols and acids such as methanol, ethanol, and formic acid. LOHCs are relatively inexpensive, readily available, and stable under ambient conditions.
When treated with appropriately designed catalysts, the hydrogen content of such molecules may be released in the form of H2, and ideally whenever and wherever H2 is needed, for example, in hydrogen fuel cells and hydrogen cars. Therefore, the development of catalysts capable of effectively catalyzing the production of H2 from LOHCs is of tremendous energy and environmental ramifications.
Chair Professor Zhiping Zheng’s research team from the Department of Chemistry at the Southern University of Science and Technology (SUSTech) has recently made significant progress toward this goal. Specifically, the researchers succeeded in extending the Noble Prize-winning Grubbs catalysts for the efficient dehydrogenation of LOHCs. Such an application goes beyond their well-established uses for olefin metathesis reactions. Their findings are detailed in a recent series of research articles.

The first paper, entitled “New Tricks for an Old Dog: Grubbs Catalysts Enable Efficient Hydrogen Production from Aqueous-Phase Methanol Reforming,” was published in ACS Catal. It focuses on a new application of the prize-winning Grubbs catalysts for hydrogen production from aqueous-phase methanol-reforming under easily achievable conditions (1 atm, <100 °C) with negligible CO formation (Figure 1).
It is also the very first ruthenium catalyst demonstrated for this capacity without needing a multidentate and nitrogen-donor ligand. Moreover, the unusual reaction mechanism involving a unique 6-m-r (or 8-m-r) methanol-assisted metathesis pathway, rather than the common metal-ligand cooperation mechanism, was proposed for the Grubbs catalysts without a non-innocent ligand based on combined mechanistic and systematic DFT studies in collaboration with Prof. Lung Wa Chung’s team.
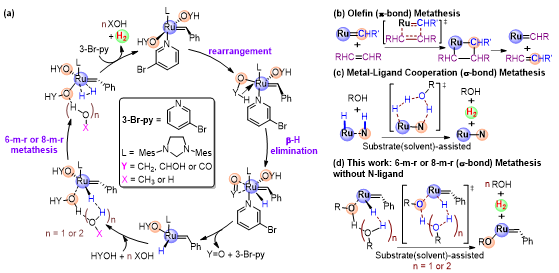
Figure 1. (a) Proposed mechanism of aqueous-phase methanol-reforming by Grubbs 3rd generation catalyst. (b-d) Comparison of reaction mechanisms: (b) Classical 4-m-r olefin (π-bond) metathesis. (c) 6-m-r nitrogen-mediated H2 evolution via metal-ligand cooperation (σ-bond) metathesis with non-innocent ligands. (d) Our proposed alternative 6-m-r or 8-m-r methanol-assisted (σ-bond) metathesis pathway responsible for the H2 evolution catalyzed by the Grubbs catalysts with innocent ligands.
Qian Wang and Jialing Lan, two joint Ph.D. students of SUSTech and the Harbin Institute of Technology, are the co-first authors of this paper. Research Assistant Prof. Lei Qin, Prof. Lung Wa Chung, and Prof. Zhiping Zheng are the co-corresponding authors.
The second paper, entitled “Hydrogen Production via Aqueous-Phase Reforming of Ethanol Catalyzed by Ruthenium Alkylidene Complexes,” was recently published in Organometallics. A series of ruthenium alkylidene complexes were tested for aqueous-phase reforming of ethanol at readily achievable temperatures (< 100 °C) (Figure 2).
The best result was obtained using Grubbs catalyst M207, producing a maximum turnover number (TON) of 47,295 with a five-day reaction, ranked as the second-largest known for homogeneously catalyzed ethanol dehydrogenation. Kinetic experiments confirmed that the reforming process undergoes a second-order kinetics reaction, with both ethanol and water contributing to H2 production.
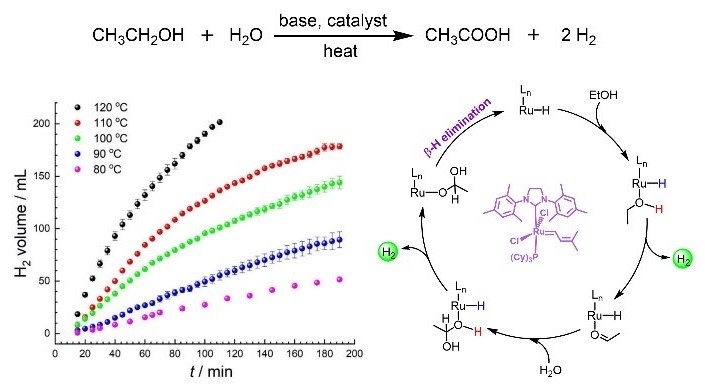
Figure 2. Hydrogen production from aqueous-phase ethanol reforming by ruthenium alkylidene complexes
Qian Wang, a joint Ph.D. student of SUSTech and the Harbin Institute of Technology, is the first author of this paper. Research Assistant Prof. Lei Qin and Prof. Zhiping Zheng are the co-corresponding authors.
The third paper, entitled “Formic Acid Dehydrogenation for Hydrogen Production Promoted by Grubbs and Hoveyda-Grubbs Catalysts,” was published in the Chinese Journal of Chemistry as a successive work. Three Grubbs catalysts and two Hoveyda-Grubbs catalysts were screened for the production of H2 by dehydrogenation of formic acid (Figure 3).
The best result was achieved with the use of the first-generation Hoveyda-Grubbs catalyst. Notably, the hydrogen generation reaction is highly pH-dependent, stemming from the transformation of divergent rate-limiting steps under different conditions. A detailed reaction mechanism was proposed based on the 1H NMR and the high-resolution mass spectroscopy data.
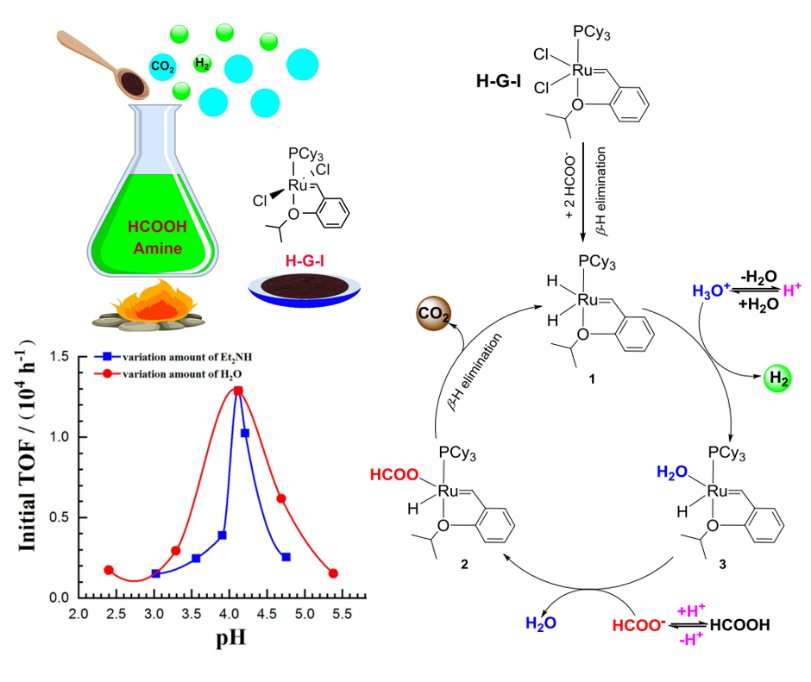
Figure 3. Hydrogen production from formic acid decomposition by the first Hoveyda-Grubbs Catalyst
Qian Wang is the first author of this paper. Research Assistant Prof. Lei Qin and Prof. Zhiping Zheng are the co-corresponding authors.
The above studies were supported by the SUSTech Presidential Postdoctoral Fellowship, Basic and Applied Basic Research Programs of Guangdong Province, Postdoctoral Scientific Research Fund for Staying in Shenzhen, Shenzhen Nobel Prize Scientists Laboratory Project, and Guangdong Provincial Key Laboratory of Catalysis. The authors also acknowledge the assistance of the SUSTech Core Research Facilities.
Paper links (In order of appearance above):
ACS Catalysis: https://pubs.acs.org/doi/10.1021/acscatal.1c05369
Organometallics: https://doi.org/10.1021/acs.organomet.1c00555
Chinese Journal of Chemistry: https://doi.org/10.1002/cjoc.202000749
To read all stories about SUSTech science, subscribe to the monthly SUSTech Newsletter.
Proofread ByAdrian Cremin, Yingying XIA
Photo By