Taurine, one of the most abundant amino acids in mammals, is widely distributed throughout various cells and tissues, including the brain, retina, leukocytes, heart, and kidneys. It plays a crucial role in physiological processes such as neural development, retinal health, immune regulation, aging and obesity, and metabolic balance. Furthermore, taurine exhibits neuroprotective and antitoxic effects in various neurodegenerative diseases, such as Parkinson’s disease, Alzheimer’s disease, and Huntington’s disease. Deficiency of taurine can cause retinal diseases and mitochondrial dysfunction, which, in severe cases may lead to blindness and dilated cardiomyopathy in humans. The uptake of taurine in human cells is mainly regulated by TauT.
TauT, a member of the solute carrier 6 (SLC6) family, shares architectural and mechanistic properties with other SLC6 members. It plays a crucial role in human physiological health, as evidenced by its protective function against oxidative stress in the Blood-Testis Barrier (BTB), thereby improving outcomes in male infertility treatment. In the inner blood-retinal barrier (BRB), TauT regulates retinal osmolarity, which is essential for maintaining retinal health. Furthermore, TauT has been implicated in cancer progression, with overexpression in gastric cancer cells leading to taurine deficiency in the tumor microenvironment and inducing T cell death and dysfunction. TauT is also associated with colorectal cancer, playing a significant role in the survival and maintenance of colorectal cancer stem cells, tumor initiation, hunger tolerance, and multidrug resistance.
TauT may serve as a pivotal target for anticancer therapies. Several compounds reported to interact with TauT are substrate analogues, such as β-alanine (β-Ala), guanidinoacetate (GAA), and γ-aminobutyric acid (GABA), as well as inhibitors, including nipecotic acid, imidazole-4-acetic acid (I4AA), piperidine-4-sulfonic acid (P4S), guanidinoethyl sulfonate (GES), 5-aminovaleric acid (5AVA), and homotaurine (Htau). Although many compounds targeting TauT have been reported, determining the high-resolution structure of TauT may be beneficial for the development of potent and selective inhibitors with anti-cancer properties.
A research team led by Assistant Professor Kaige Yan from the Departments of Chemistry and Biology, School of Life Sciences at the Southern University of Science and Technology (SUSTech) has uncovered the molecular architecture of human TauT. Their comprehensive structural analysis deepens the understanding of taurine release, substrate specificity, inhibitor recognition, and inhibition mechanisms of TauT, highlighting significant advancements for structure-based design of drugs targeting TauT.
Their work, “Molecular basis of human taurine transporter uptake and inhibition,” has been published in the journal Nature Communications.
The team successfully determined the high-resolution cryo-electron microscopy structures of TauT bound to different substrates or substrate analogues, revealing the specific binding patterns of TauT to these small-molecule substrates. When TauT binds to the substrate taurine or its analogue β-alanine, it is captured in an occluded conformation, while the substrate analogue guanidinoacetate and γ-aminobutyric acid stabilize TauT in an intermediate conformation. Combining the structural studies with biochemical analyses, their research revealed that taurine and its analogues bind to the central binding cavity of TauT. They also disclosed the core roles of two key amino acid residues, Leu134 and Glu406, in the substrate specificity of TauT through biochemical analysis and structural comparison, providing a new perspective for understanding the substrate selectivity of the GABA transporter subfamily.
Their study determined the cryo-electron microscopy structures of TauT in combination with six different inhibitors. The results showed that these inhibitors occupied the substrate binding cavity and stabilized TauT in different conformations, thereby blocking the transport of the substrate taurine. Except for the inhibitor GES, which stabilized TauT in the occluded conformation, the other five inhibitors stabilized TauT in the inward-open conformation. They further compared and analyzed these structures with the TauT structure bound to taurine, indicating that the larger volume and longer carbon linker structure characteristics of the inhibitors would hinder the conformational changes required for TauT transport, thereby inhibiting the substrate transport process.
The researchers conducted a structural and sequence comparison analysis, hypothesizing that the non-conservative residues in this binding pocket might influence the different conformational preferences of structures of TauT and the family member transporter GAT1 bound to the inhibitor nipecotic acid. Their findings provide important clues for designing efficient and specific TauT inhibitors.
Additionally, they revealed the molecular mechanism of TauT transport. The N-terminal residue Arg41 had extensive interactions with TM6 and TM8, closing the intracellular channel and stabilizing the occluded conformation of TauT. In this conformation, two sodium ions and one chloride ion could be clearly seen. In contrast, in the intermediate conformation, TM1 of TauT unwound a small-angle helix, and the sodium ion at the Na2 site exhibited a solvation phenomenon. When TauT transformed into an inwardly open conformation, TM1a unwound the helix, disrupting the interaction of the intracellular channel and causing the release of taurine from the binding pocket. The conservation density of the chloride ion binding site confirmed the dependence of taurine transport on chloride ions. Their study depicted the transition of TauT from the occluded state to the inwardly open state, advancing the understanding of the mechanism of taurine release.
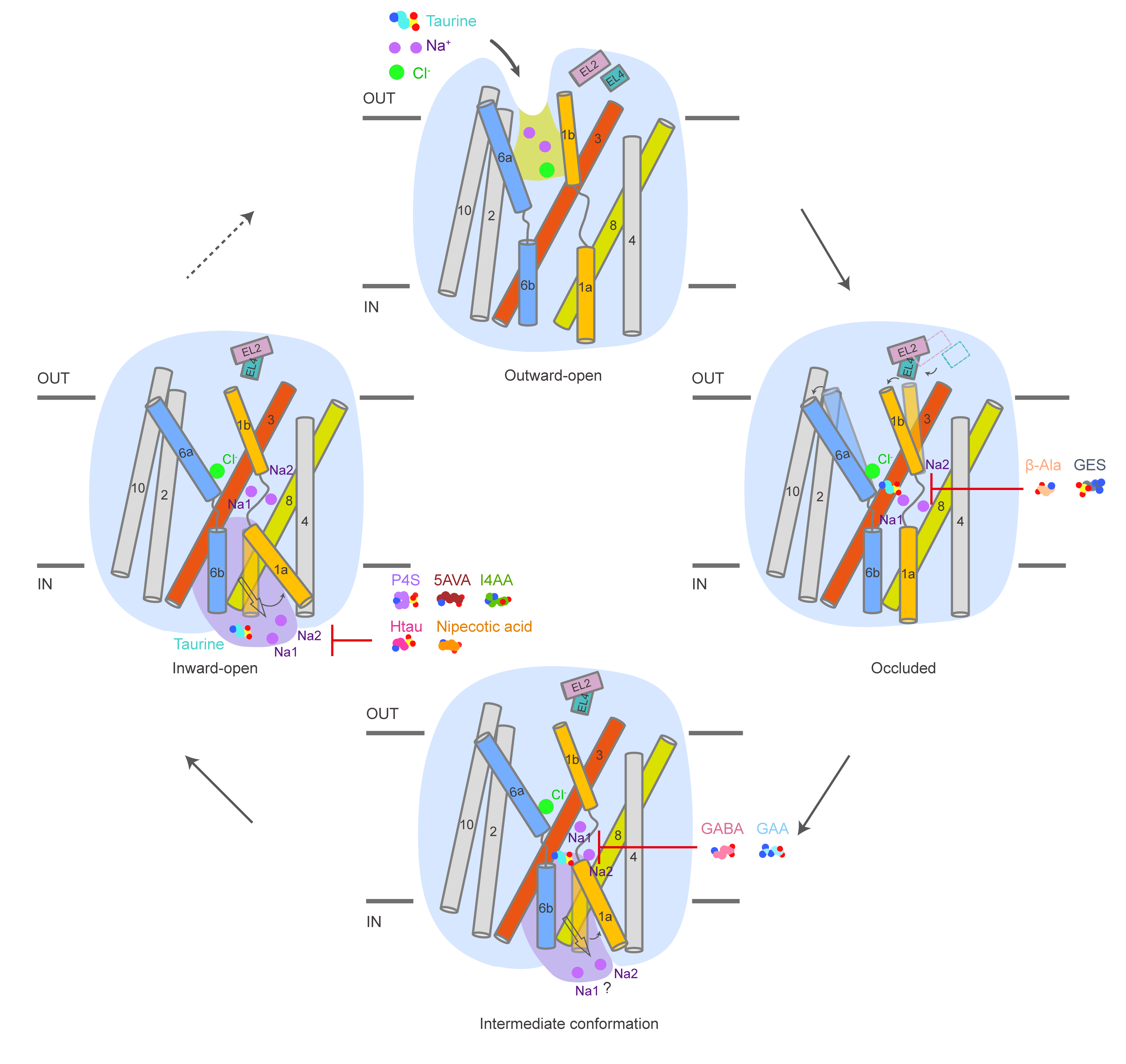
Figure 1. The transport and inhibition mechanism of human TauT
Ph.D. student Bowen Du and master’s student Jiaying Xie from the School of Life Sciences at SUSTech, along with postdoctoral researcher Lili Cheng from the School of Pharmaceutical Sciences at Tsinghua University, are the co-first authors of the paper. Assistant Professor Kaige Yan from SUSTech and Professor Ligong Chen from Tsinghua University serve as the corresponding authors.
Paper link: https://www.nature.com/articles/s41467-025-62857-w
To read all stories about SUSTech science, subscribe to the monthly SUSTech Newsletter.
Proofread ByAdrian Cremin, Yifei REN
Photo BySchool of Life Sciences