Recently, the Research Group led by Jiang Wei under Chemistry Department solved the problem on “selective recognition of polar molecules with hydrogen bonds in water”, developing a simple method to detect persistent pollutant – 1,4-dioxane in underground water. Such result was published online in Journal of the American Chemical Society (JACS) on October 28, 2016 (Impact Factor = 13.038).
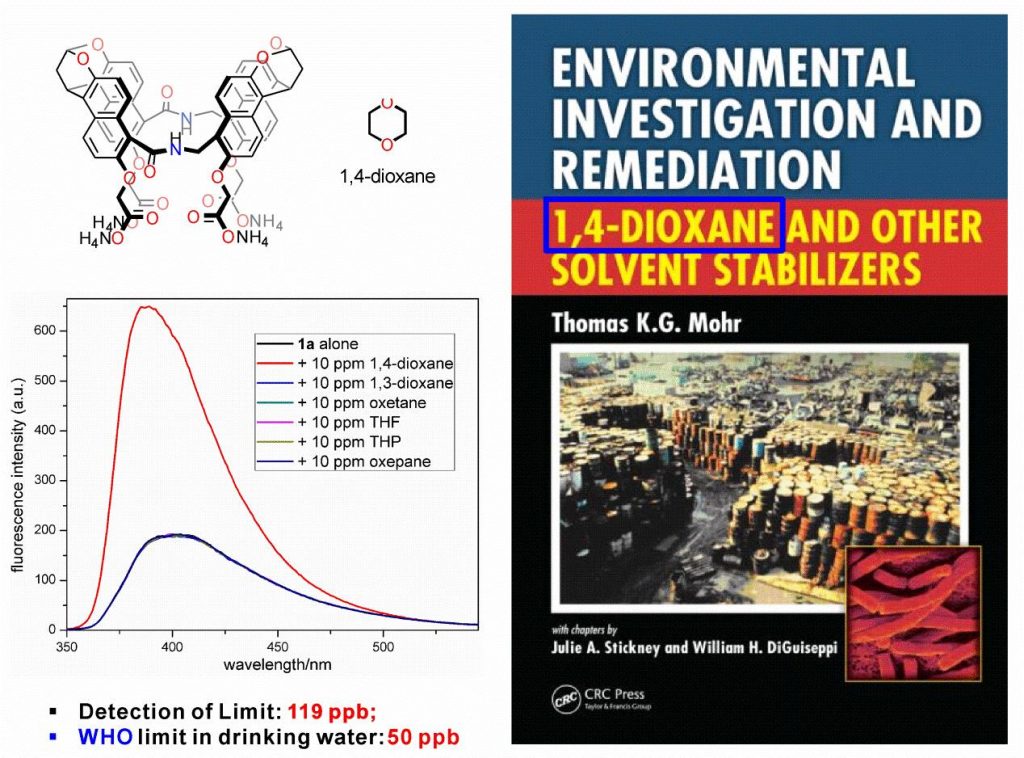
Selective recognition of polar molecules in water is an acknowledged problem in the field of supramolecular chemistry, which is owing to 1) severe salvation of polar molecules in water and 2) high difficulty of molecular recognition with hydrogen bond in water. Inspired from the structure of biological receptor, Jiang Wei’s Group proposed the concept of “inner modification of molecular tubes”, namely to implant the binding site of hydrogen bond into the hydrophobic deep cavity, which provides non-polar environment for weak interactions concerning hydrogen bond and avoids the competitions among water molecules. The synergy between hydrophobic effect and hydrogen bond will significantly enhance the interactions between host and guest molecules. According to the theory, the Group figured out that the modified molecular tubes in amide are able to selectively recognize polar solvent molecules in water, such as 1,4-dioxane, DMF, DSMO, acetone and tetrahydrofuran. The bonding constant to 1,4-dioxane climbs to 104 M-1, which is, so far, the strongest value to this highly hydrophilic molecule. The tests via X-ray single-crystal diffraction, Isothermal Titration Calorimetry and nuclear magnetism titration proved the significance of hydrogen bond in molecular recognition. This research perfectly solved the problem of “high difficulty to selectively recognize polar molecules in water with hydrogen bond”.
As the mother nucleus structure of dioxin, 1,4-dioxane is usually used as the stabilizer of chloro-substituted organic solvents and mainly generated from the cosmetic manufacturing as a by-product. International Agency for Research on Caner classifies it as Group 2B. 1,4-dioxane is highly hydrophilic and cannot be absorbed by soil. Once poured outside, it will be gradually accumulated in water as a persistent pollutant in the underground water. Since scientists recognized the hazards of 1,4-dioxane in the 1990s, several monographs have been published. Due to its features, the detection and elimination are very difficult. The traditional detection methods – solid-phase extraction with GC-MS are complicated, time-consuming and costly. The inner modified molecular tubes of the Group, after addition with 1,4-dioxane, can achieve fluorescence enhancement and be applicable for the detection of 1,4-dioxane in the underground water, with a limit of detection (LOD) to 120 ppb in maximum, which is very close to the safety limit (50 ppb) of 1,4-dioxane in drinking water specified by World Health Organization. It is worth mentioning that the fluorescence detection method is easy and convenience without any pre-treatment on samples. For now, the Group is now striving to improve the acceptor molecule, with an aim to reduce the LOD of 1,4-dioxane and find an efficient way to remove 1,4-dioxane from water.
The group’s research focuses on the designing and synthesis of new supramolecular hosts and relevant applications in molecular machines, analysis & detection, intelligent materials, chemical solubilisation and delivery, etc. After the establishment, the Group published 13 papers in SUSTech, including one Chem. Soc. Rev., one JACS, two Chem. Sci., four as covers on Chem. Sci and Chem. Commun. and three highlighted by Chemical Science Blog and ACS Cutting-Edge Chemistry.
Paper link: http://pubs.acs.org/doi/abs/10.1021/jacs.6b09472
Proofread By
Photo By