New research conducted by an international set of researchers may have found a new method of developing medical-grade chemical without using heavy metals. It could see drug development become cheaper, less dangerous, and better for the environment.
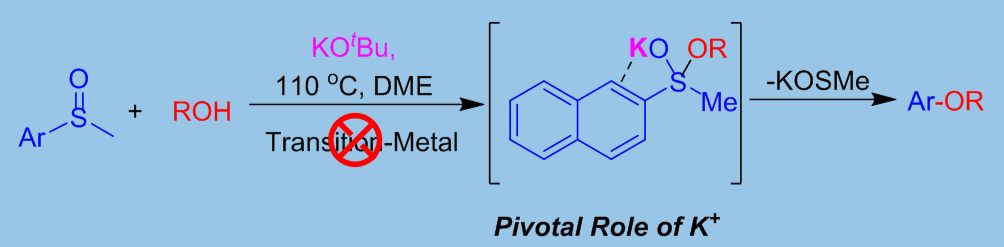
Fig. 1 Transition-metal-free C-O coupling of sulfoxides and alcohols via C-S bond activation.
On June 8, the research teams of Assistant Professor Tiezheng Jia (Chemistry) and Professor Marisa Kozlowski (University of Pennsylvania Department of Chemistry) collaborated to publish a paper in the high-impact academic journal, Nature Communications. Their paper was titled “Transition-metal-free formal cross-coupling of aryl methyl sulfoxides and alcohols via nucleophilic activation of C-S bond.” The editors of Nature Communications have nominated the article as one of its highlights for recent research on organic chemistry and chemical biology.
Ethers are vital functional groups, which are widely spread in natural products as well as artificial materials. These products and materials include food additives, cosmetics, and pharmaceuticals. It had attracted lots of attention from both academia and industry, especially in the synthesis of pharmaceutical products.
Transition-metal catalyzed C-O cross-couplings between aryl halides has become one of the most fundamental approaches. However, heavy metals, like copper (Cu), nickel (Ni), palladium (Pd), ruthenium (Ru) or rhodium (Rh), are often used. These heavy metals are toxic, bad for the environment, and costly. Dealing with the residue of the transition metals presents an enormous problem for the synthesis of pharmaceutical products.
The researchers developed a novel methodology of potassium tert-butoxide (KtOBu) promoted C-O cross-coupling between sulfoxides and alcohols. Their method avoided using transition-metals. The C-S bond of sulfoxide could be activated so that a series of diverse ether were synthesized conveniently and effectively.
Two drug molecules were successfully prepared by using this transition-metal free coupling protocol as a critical step. The positive outcome has emphasized its potential use in medicinal chemistry and drug development.

Fig. 2 Synthetic application of pharmaceuticals.
A computational chemistry study showed the pivotal role of potassium cation and provides a new view of cross-coupling processes via C-S bond activation.

Fig. 3 Proposed reaction mechanism and DFT research.
The co-first authors of the paper are Guolin Li (SUSTech) and Dr. Yexenia Nieves-Quinones (UPenn). The co-correspondent authors are Assistant Professor Tiezheng Jia and Professor Marisa Kozlowski. Additional contributions came from Northwest University.
The authors received support from the Shenzhen Nobel Prize Scientists Laboratory Project, the Science and Technology Innovation Commission of Shenzhen Municipality, the Guangdong Provincial Key Laboratory of Catalysis, the SUSTech startup funds, and the US NIH.
Proofread ByYingying XIA
Photo ByDepartment of Chemistry, Yan QIU