Researchers at Southern University of Science and Technology (SUSTech) have made significant progress in engineering novel alloy nanomaterials. Their research opens up an effective strategy for boosting catalytic reactions by creating atom-doped amorphous oxide surfaces derived from novel bimetallic alloys, further enhancing opportunities for the conversion of greenhouse gases to renewable fuels.
On July 11, Professor Zewei Quan (Chemistry) has led his research group to publish a breakthrough paper in the high-impact academic journal, Advanced Materials (IF = 27.4). The paper was titled “Novel Bi-Doped Amorphous SnOx Nanoshells for Efficient Electrochemical CO2 Reduction into Formate at Low Overpotentials”.
Engineering novel bimetallic materials provide intriguing catalytic properties to boost the catalytic reactions. Low cost, non-toxic and environmental-friendly tin (Sn) and bismuth (Bi) are good candidates for electrocatalysis. However, the large lattice mismatch (> 22%), easy phase separation, and oxidization, as well as low solubility (<2 at%) between Sn and Bi, means that preparing phase-pure Sn1-xBix alloys remain a significant challenge.
The research group reported the first synthesis of homogeneous Sn1-xBix alloy nanoparticles (x up to 0.20) with tunable composition under the water-free and oxygen-free conditions, in which the concentration of Bi is much higher than previous reports (<2 at%).
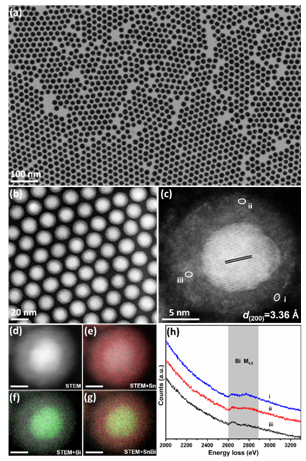
Structural characterization of core-shell Sn0.80Bi0.20@Bi-SnOx nanoparticles
In general, Sn and Bi are both air-sensitive and readily oxidized metals. When Sn1-xBix alloy nanocrystals are exposed to air, Sn and Bi atoms in the surface layer will oxidize to form amorphous nanoshells. Using different characterization methods, the research group determined that the oxide nanoshell was a zero-valent Bi atom doped amorphous SnOx. The absence of Bi oxide in the nanoshells is due to the competing oxidation process of the metallic phases.
During the competitive oxidation process, Sn atoms with a higher oxygen affinity oxidize to form amorphous SnOx while relatively inert Bi atoms remain stable. Due to the interaction between Sn and Bi atoms in the original Sn1-xBix alloy lattice, Bi atoms are directly involved in this transformation process. They are anchored in the as-formed amorphous SnOx, before obtaining the novel zero-valent Bi atom doped amorphous SnOx nanoshell (Bi-SnOx).
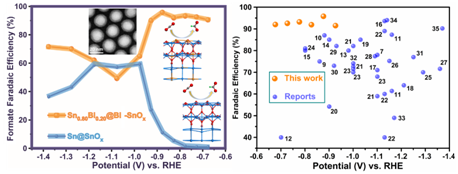
Electrochemical CO2 reduction performance of Sn0.80Bi0.20@Bi-SnOx
The research group also investigated the performance of Bi-SnOx nanoshells in the electrochemical reduction of CO2. The state-of-the-art Bi-SnOx nanoshells boost the production of formate with high Faradaic efficiencies (>90%) over a wide potential window (-0.67 to -0.92 V vs. RHE). They outperform current tin oxide catalysts, revealing that the incorporation of Bi atoms into Sn species facilitates formate production by suppressing the formation of H2 and CO.
In this work, Professor Zewei Quan is the corresponding author. Department of Chemistry research assistant Qi Yang is the first author of this paper. This work received additional support from Professor Pei Kang Shen from the College of Chemistry and Chemical Engineering of Guangxi University and SUSTech Associate Professor Lele Duan.
This work is supported by the National Natural Science Foundation of China, the Ministry of Science and Technology, the Ministry of Education Key Laboratory, and other projects.
Paper link: https://onlinelibrary.wiley.com/doi/full/10.1002/adma.202002822
Proofread ByYingying XIA
Photo ByDepartment of Chemistry, Yan QIU