Associate Professor Chuan He’s team of the Department of Chemistry at the Southern University of Science and Technology (SUSTech) has recently made crucial breakthroughs in the field of chiral organosilicon chemistry. Their findings were published in the Journal of the American Chemical Society, Angewandte Chemie International Edition, Nature Communications, etc., which provide a new direction for the synthesis and application of chiral organosilicon compounds.

Due to the unique chemical, physical and biological properties, organosilicon compounds are widely used in aviation, construction, coatings, electronics and electricity, medicine and medical treatment, organic materials, and many other fields. However, organic silicon was omitted in biological evolution. Therefore, chiral organosilicon compounds are not found in nature.
The creation of silicon-stereogenic centers in enantioenriched forms has been significantly less explored due to their intrinsic properties and the enantioselective synthesis of silicon-stereogenic silanes has always been a challenge. In recent years, these interesting chiral organosilicon compounds have found various valuable applications in the fields of synthetic chemistry, materials science, pharmaceutical chemistry, and life science. Therefore, the exploration and development of efficient catalytic methods that enable the construction of new scaffold silicon-stereogenic silanes with high enantioselectivities are highly warranted.
Since 2018, Chuan He’s research group has carried out a series of research work focusing on the development of new asymmetric catalytic methods for the efficient construction of silicon-stereogenic compounds, as well as the exploration of their functions and applications. They have achieved many innovative research results with distinctive features.
Starting from simple dihydrosilanes and olefins, a tandem highly enantioselective C–H silylation/alkene hydrosilylation methodology was developed. This enables the streamlined construction of the architecturally complex and functionally diverse enantioenriched tetra-substituted silicon-stereogenic silanes in a single step with good to excellent yields and enantioselectivities (Figure 1). With rational design, various enantioenriched silicon-stereogenic silanes, including 9-silafluorenes, Si-bridged ladder compounds, and benzosilolometallocenes can be synthesized.
This work was published in the Journal of the American Chemical Society. The paper was entitled “Streamlined Construction of Silicon-Stereogenic Silanes by Tandem Enantioselective C–H Silylation/Alkene Hydrosilylation.” The co-first authors are Postdoctoral Fellows Delong Mu and Wei Yuan from the Department of Chemistry at SUSTech.
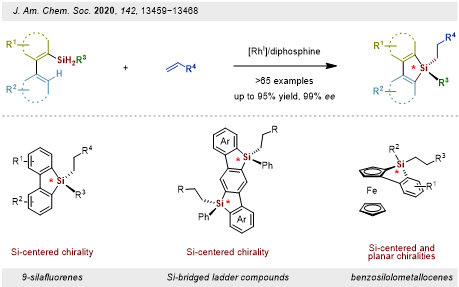
Figure 1. Construction of tetra-substituted silicon-stereogenic siloles
Continuous work unprecedentedly developed an enantioselective aliphatic C–H silylation/alkene hydrosilylation methodology for the efficient synthesis of silicon-stereogenic dihydrobenzosiloles. A wide range of dihydrosilanes and alkenes displaying various functional groups are compatible with this process, giving access to a variety of highly functionalized silicon-stereogenic dihydrobenzosiloles in good to excellent yields and enantioselectivities (Figure 2).
These results were published in Angewandte Chemie International Edition. The paper was entitled “Enantioselective Silylation of Aliphatic C–H Bonds for the Synthesis of Silicon-Stereogenic Dihydrobenzosiloles.” The first author is Postdoctoral Fellow Bo Yang from the Department of Chemistry at SUSTech.
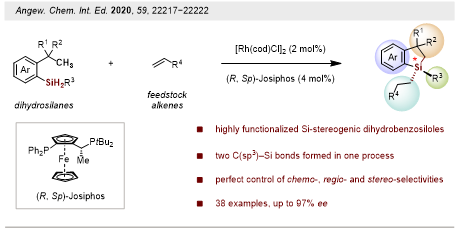
Figure 2. Construction of tetra-substituted silicon-stereogenic dihydrobenzosiloles
Moreover, asymmetric synthesis of silicon-stereogenic monohydrosilanes by rhodium-catalyzed dehydrogenative C–H silylation was developed. The process is suitable for the synthesis of various asymmetrically trisubstituted 1H-benzosiloles and 1H-benzosilolometallocenes in good yields with excellent chemo-, regio-, and stereo-control (Figure 3).
This work was published in Organic Letters. The paper was entitled “Asymmetric Synthesis of Silicon-Stereogenic Monohydrosilanes by Dehydrogenative C–H Silylation”. The co-first authors are Postdoctoral Fellow Wei Yuan and Master’s student Lijun You from the Department of Chemistry at SUSTech.
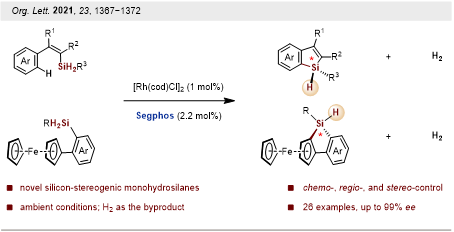
Figure 3. Synthesis of silicon-stereogenic monohydrosilanes
The group further developed a rhodium-catalyzed enantioselective dehydrogenative C–H silylation methodology, which enables the construction of a wide range of six- and seven-membered triorgano-substituted silicon-stereogenic heterocycles in good to excellent yields and enantioselectivities (Figure 4). Most of these silicon-bridged heterocycles exhibit bright blue fluorescence. These previously inaccessible asymmetrically silicon-stereogenic heterocycles will find widespread use among synthetic chemistry and optoelectronic materials science.
These results were published in Nature Communications. The paper was entitled “Enantioselective Construction of Six- and Seven-Membered Triorgano-Substituted Silicon-Stereogenic Heterocycles.” The co-first authors are Postdoctoral Fellows Shuyou Chen and Delong Mu from the Department of Chemistry at SUSTech.
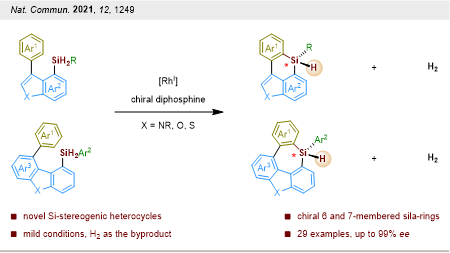
Figure 4. Construction of six- and seven-membered silicon-stereogenic heterocycles
It was also revealed that in the presence of Rh (I) catalyst with chiral diphosphine ligand, a wide range of dihydrodibenzosilines containing both silicon-central and axial chiralities are conveniently constructed in excellent enantioselectivities via dehydrogenative Csp3–H silylation (Figure 5). Absolute configuration analysis by single-crystal X-ray structures revealed a novel silicon-central to axial chirality relay phenomenon, which will inspire further research in the field of asymmetric catalysis and chiroptical materials.
These results were published in Angewandte Chemie International Edition. The paper was entitled “Catalytic Asymmetric Synthesis of Silicon-Stereogenic Dihydrodibenzosilines: Silicon-Central to Axial Chirality Relay.” The co-first authors are the Ph.D. student Yonghong Guo and Postdoctoral Fellow Meng-Meng Liu from the Department of Chemistry at SUSTech.
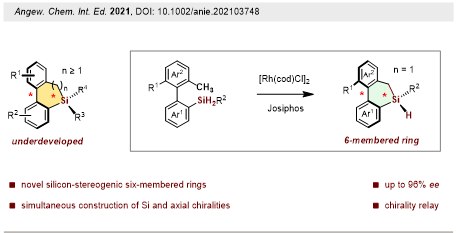
Figure 5. Chiral Silicon-central to axial chirality relay
With the success in intramolecular reactions, the group further studied the more challenging intermolecular dehydrogenative coupling for the construction of silicon-stereogenic silanes. This process undergoes a direct intermolecular dehydrogenative Si−O coupling between dihydrosilanes with silanols or alcohols, giving access to a variety of highly functionalized chiral siloxanes and alkoxysilanes in decent yields with excellent stereocontrol, that significantly expand the chemical space of the silicon-centered chiral molecules. (Figure 6). Further utility of this process was illustrated by the construction of CPL-active (circularly polarized luminescence) silicon-stereogenic alkoxysilane small organic molecules, which could have great potential application prospects in chiral organic optoelectronic materials.
This work was published in the Journal of the American Chemical Society. The paper was entitled “Catalytic Enantioselective Dehydrogenative Si–O Coupling to Access Chiroptical Silicon-Stereogenic Siloxanes and Alkoxysilanes.” The co-first authors are Ph.D. student Jiefeng Zhu and Postdoctoral Fellow Shuyou Chen from the Department of Chemistry at SUSTech.
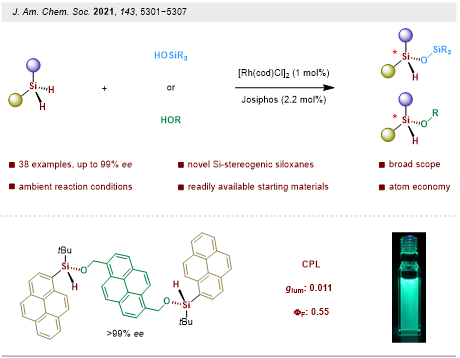
Figure 6. Construction of Si-Stereogenic siloxanes and alkoxysilanes
Meanwhile, an efficient electrochemical radical silyl-oxygenation of electron-deficient alkenes is also demonstrated, which gives access to a variety of new highly functionalized silicon-containing molecules, including β-silyl-cyanohydrin derivatives in good yields with excellent chemo- and regio-selectivity (Figure 7). This electrochemical radical silylation process conducts under mild conditions without the use of transition metal catalyst or chemical oxidant and exhibits a wide scope of substrate silanes with high functional group tolerance. The ability to access silyl radicals using electrochemical Si−H activation offers new perspectives for the synthesis of valuable organosilicon compounds in a sustainable and green manner.
These results were published in Angewandte Chemie International Edition. The paper was entitled “Electrochemical Radical Silyl-Oxygenation of Activated Alkenes.” The co-first authors are Senior Research Scholar Jie Ke and Postdoctoral Fellow Wentan Liu from the Department of Chemistry at SUSTech.

Figure 7. Electrochemical radical silyl-oxygenation of alkenes
Associate Professor Chuan He of the Department of Chemistry at SUSTech is the sole corresponding author of all the above papers, with SUSTech as the sole communication unit. The above researches were financially supported by the National Natural Science Foundation of China (NSFC), Shenzhen Science, Technology and Innovation Commission (SZSTI), Shenzhen Nobel Prize Scientist Laboratory Project, and the Guangdong Provincial Key Laboratory of Catalysis.
They also acknowledge the Center for Computational Science and Engineering at SUSTech for its theoretical calculation support, and the SUSTech Core Research Facilities for its technical support. A special thanks and recognition was also mentioned for Associate Professor Peiyuan Yu, Professor Feng He, Professor Wei Lu, Associate Professor Qin Yin, Research Assistant Professor Xiao-Yong Chang, Visiting Scholar Yingzi Li, Research Assistant Professor Chao Zou, and Postdoctoral Fellow Tingxing Zhao, all of whom are faculty members of the Department of Chemistry at SUSTech, for their help throughout the research.
Paper links (In order of the news above):
Journal of the American Chemical Society: https://pubs.acs.org/doi/10.1021/jacs.0c04863
Angewandte Chemie International Edition: https://onlinelibrary.wiley.com/doi/10.1002/anie.202009912
Organic Letters: https://pubs.acs.org/doi/10.1021/acs.orglett.1c00029
Nature Communications: https://www.nature.com/articles/s41467-021-21489-6
Angewandte Chemie International Edition: https://onlinelibrary.wiley.com/doi/10.1002/anie.202103748
Journal of the American Chemical Society: https://pubs.acs.org/doi/10.1021/jacs.1c01106
Angewandte Chemie International Edition: https://onlinelibrary.wiley.com/doi/10.1002/anie.202016620
Professor Chuan He’s webpage: https://faculty.sustech.edu.cn/hec/en/
Proofread ByAdrian Cremin, Yingying XIA
Photo By