Prostaglandins are among the most important natural isolates owing to their broad range of bioactivities and unique structures. Currently, there are more than 20 prostaglandins marketed worldwide. The development of efficient methods to synthesize PGs has been a goal of synthetic chemists for almost 50 years. However, current methods for the synthesis of PGs still suffer from low yields and lengthy steps. A concise and scalable synthetic route for more efficient and green production of PGs and related drugs is highly desirable.
Professor Xumu Zhang from the Department of Chemistry at the Southern University of Science and Technology (SUSTech) and Associate Professor Gen-Qiang Chen from the SUSTech Academy for Advanced Interdisciplinary Studies realized the highly efficient and asymmetric synthesis of prostaglandins using the self-developed enyne cycloisomerization and asymmetric hydrogenation reaction as the key steps, which significantly improved the synthetic efficiency. Their result was published in Nature Chemistry, entitled “Concise, Scalable and Enantioselective Total Synthesis of Prostaglandins.”

PGF2α is one of the most complex compounds in the prostaglandin family. Its core structure consists of a cyclopentane skeleton with four continuous chiral centers and two side chains (Figure 1a). In 1964, E. J. Corey realized the first total synthesis of PGF2α. Then, many famous organic chemists like Woodward, Stork, Noyori, Danishefsky, Aggarwal, Baran, Grubbs and Chen, etc., have also made significant contributions in the synthesis of prostaglandins.
Although substantial progress has been made in the synthesis of prostaglandins and several synthetic routes have been developed, these existing synthetic routes are not suitable for industrial synthesis. At present, the industrial synthesis of prostaglandins still relies on Corey lactone (10), which can be synthesized from cyclopentadiene 9 through nine steps. Several steps are needed from the Corey lactone to obtain prostaglandins, and some prostaglandins require more than ten steps to synthesize (Figure 1b). In this work, Xumu Zhang and his colleagues designed and synthesized a key intermediate 15 to synthesize prostaglandins, from which PGs can be synthesized in only two steps. This intermediate can be synthesized in four steps from the readily available β-ketoamide 11 (Figure 1c).
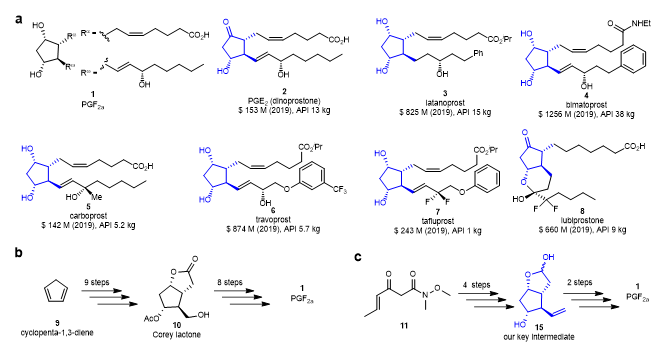
Figure 1. PGs and their synthetic methods. (a) Representative examples of PGs and related drugs. (b) Corey’s synthesis of PGF2α via Corey lactone 10. (c) Concise synthesis of PGF2α via key intermediate 15.
The main difficulties in the asymmetric synthesis of prostaglandins lie in the precise construction of four continuous chiral centers in the core cyclopentane skeleton and the efficient introduction of two side chains. In 2000, Professor Zhang’s research team reported the first rhodium-catalyzed cycloisomerization of 1,6-enynes, realizing the efficient construction of chiral five-membered cyclic compounds. For most substrates, the reaction can obtain very high enantioselectivity by using the common diphosphine BINAP as a chiral ligand. The enyne cycloisomerization was named “Zhang enyne cycloisomerization” in 2014 and has become one of the few chemical reactions named after a Chinese person (Figure 2).
In 2016, Professor Zhang’s team designed and synthesized a chiral tridentate ligand f-amphox (L1) based on the ferrocene framework, which exhibits excellent activity and enantioselectivity in asymmetric hydrogenation of simple ketones (up to 1,000,000 TON, up to >99% Ee) with iridium complex. The reaction can be carried out efficiently, even in solvent-free conditions (Org. Lett. 2016, 18, 2938-2941).

Figure 2. Zhang enyne cycloisomerization and its mechanism
The research team envisaged the rhodium-catalyzed isomerization of the asymmetric enyne ring as the key step to construct the chiral cyclopentane skeleton, thus achieving the asymmetric synthesis of the prostaglandin PGF2α. They performed a retrosynthetic analysis of the prostaglandin PGF2α (Fig. 3). They found that the target compound PGF2α (1) could be synthesized from the key intermediate 15 through the Grubbs olefin cross-metathesis and the Wittig reaction. The key intermediate 15 can be traced back to 14 through successive reductions and deprotections. It was evident that compound 14 is a typical product of our enyne cycloisomerization originating from 13. By nucleophilic addition, Weinreb amide 12 can be converted to compound 13. Enantioenriched compound 12 is readily accessible through asymmetric hydrogenation.
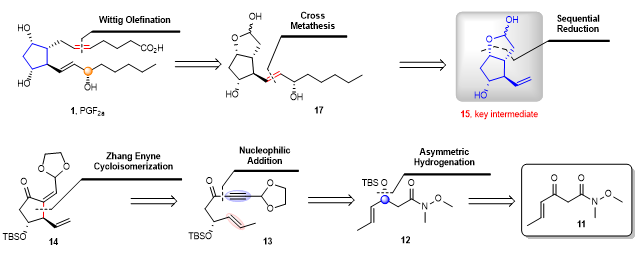
Figure 3. The retrosynthetic analysis of prostaglandins
Based on the above retrosynthetic analysis, the authors explored the synthesis of prostaglandin PGF2α with β-ketoamide 11 as the starting material (Figure 4). Using f-amphox (L1) developed by Professor Zhang’s research group as chiral ligand, β-ketoamide 11 can be enantioselectively reduced with 70% yield and 94% enantioselectivity to provide compound 12. Weinreb amide in compound 12 can be successfully converted into 1,6-enyne 13 through an addition reaction with the lithium salt of compound 18. Using rhodium as the catalyst and (S)-BINAP as chiral ligand, the asymmetric cycloisomerization of 1,6-enyne compound 13 could proceed smoothly to provide 14 with 85% yield, 98% ee, and >20/1 dr. The conjugated double bond and carbonyl group in compound 14 can be reduced by diphenyl silane and triethyl lithium-borohydride, respectively, and the key intermediate 15 or 16 can be selectively obtained by adjusting the workup of the reduced product. The stability of the intermediate 16 is greater than that of the intermediate 15, and the intermediate 16 can also be transformed into the intermediate 15 by hydrochloric acid treatment.
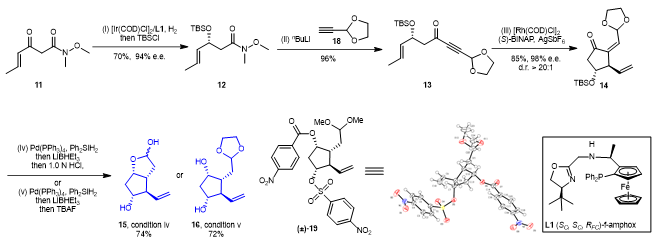
Figure 4. Efficient synthesis of the key intermediates of PGs 15 and 16
After the key intermediate 15 was successfully synthesized, the final synthesis of prostaglandins was attempted (Figure 5). Compound 17 could be obtained with the 2nd generation of Hoveyda-Grubbs catalyst, the key intermediate 15, and the chiral alcohol intermediate 19 can react with Grubbs cross-metathesis and Wittig olefination to obtain. Compound 17 can be obtained smoothly with a 15% total yield after one step of classical Wittig reaction. Using a similar synthetic route, latanoprost (3), carboprost (5), and cloprostenol (22) were also synthesized with total yields of 5.7%, 23%, and 19%, respectively. It is worth mentioning that fluprostenol (23) can be synthesized with a total yield of 27% and on a scale of 23g, and one step esterification of fluprostenol (23) leads to travoprost (6). In addition, dinoprostone (2) can also be formally synthesized from compound 11 in seven steps.
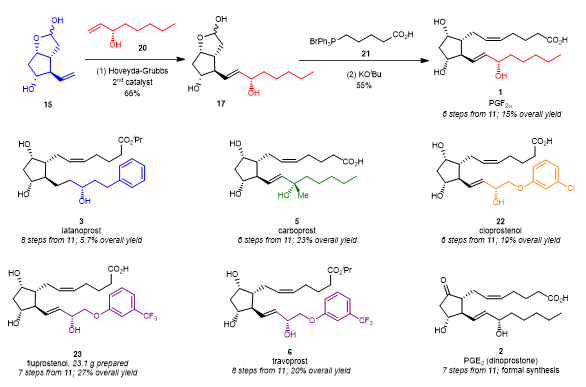
Figure 5. Completion of the total synthesis of PGs
Finally, Xumu Zhang and Gen-Qiang Chen’s research teams designed and synthesized two key intermediates to synthesize prostaglandins, 15 and 16, and realized the efficient synthesis of these intermediates by self-developed enyne cycloisomerization reaction and asymmetric hydrogenation. Based on this intermediate, the PGs can be synthesized in only two steps. This synthesis method has the atom economy, step economy, and redox economy characteristics, which provides great convenience for synthesizing prostaglandin-related drugs. It can effectively reduce the production cost of prostaglandin-related drugs and has a very important significance for the research and development of prostaglandin-related drugs.
Professor Xumu Zhang from SUSTech and Gen-Qiang Chen from the SUSTech Academy for Advanced Interdisciplinary Studies are the co-corresponding authors of this paper. Fuhao Zhang, a joint doctoral candidate of Professor Xumu Zhang at SUSTech, and Professor Yixin Lu from the National University of Singapore are the first authors of this paper.
The research work was supported by the National Natural Science Foundation of China (NSFC), Key-Area Research and Development Program of Guangdong Province, Innovative Team of Universities in Guangdong Province, Shenzhen Science and Technology Innovation Committee, and the Shenzhen Nobel Prize Laboratory projects.
Paper links:
Nature Chemistry: https://www.nature.com/articles/s41557-021-00706-1
Name Reactions (2014): https://link.springer.com/chapter/10.1007/978-3-319-03979-4_297
Proofread ByAdrian Cremin, Yingying XIA
Photo By