Transition metal-catalysed C–C cross-coupling reactions have revolutionized organic synthesis, providing an essential toolkit for the expedited synthesis of medicines, agrochemicals, and functional material molecules. With the goal of providing sustainable strategies for the synthesis of enantioenriched three-dimensional molecules, great efforts have been dedicated to the development of chiral earth-abundant first-row transition metal catalysts (Ni, Co, and Fe), which easily convert racemic alkyl electrophiles to prochiral alkyl radicals via a single-electron transfer process.
However, the enantioselective construction of all-carbon quaternary stereocenters directly starting from racemic tertiary electrophiles through radical intermediate remains largely unexplored. The major challenge arises from the steric hindrance and conformational flexibility of reactive tertiary radicals, which makes the enantioselective differentiation of three distinct carbon substituents of such species difficult.

Professor Xin-Yuan Liu’s research team from the Department of Chemistry at the Southern University of Science and Technology (SUSTech), in collaboration with researchers from Zhejiang University, have recently made a breakthrough in asymmetric radical C(sp3)–C(sp) cross-coupling to forge synthetically challenging quaternary carbon building blocks.
Their paper, entitled “Mechanism-based ligand design for copper-catalysed enantioconvergent C(sp3)–C(sp) cross-coupling of tertiary electrophiles with alkynes,” was published in Nature Chemistry, one of the most preeminent academic journals in chemistry.
In 2019, Prof. Liu’s team disclosed an anionic N,N,P-ligand/Cu catalytic system (Nat. Chem. 2019, 11, 1158), which provides a practical platform for enantioconvergent cross-coupling of secondary electrophiles and alkyne (Fig. 1b). Based on these results and further DFT calculations, the researchers proposed an outer-sphere radical group transfer pathway for tertiary radical (Fig. 1c). As such, they conceptually and rationally designed a series of anionic N,N,N-ligands featuring a long spreading side arm for enantioconvergent cross-coupling of tertiary electrophiles (Fig. 1d).
They disclosed a set of amide-derived anionic N,N,N-ligands for realizing a general copper-catalysed enantioconvergent radical C(sp3)–C(sp) cross-coupling of racemic tertiary alkyl halides with terminal alkynes under mild reaction conditions (Fig. 1e). This protocol exhibits a remarkably broad scope (87 examples) with respect to both coupling partners. It covers a variety of (hetero)aryl-, alkenyl- and alkyl- terminal alkynes, as well as both acyclic racemic tertiary α-haloamides and cyclic racemic α-halo-β-lactams with good functional group compatibility. Additionally, it provides a practical platform for the construction of chiral C(sp3)–C(sp), C(sp3)–C(sp2), and C(sp3)–C(sp2) bonds leading to the expedient assembly of diversely synthetically challenging quaternary carbon building blocks of interest in pharmaceutical research and other areas.
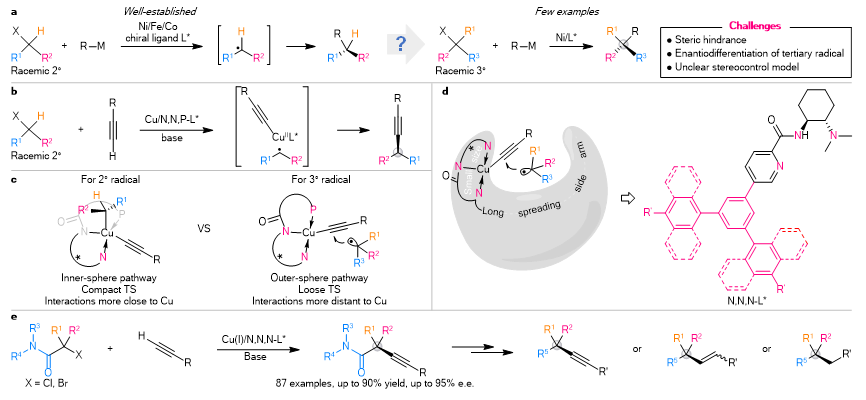
Figure 1. Motivation and design of Cu-catalysed enantioconvergent cross-coupling of racemic tertiary alkyl halides with terminal alkynes. (a) The state of the art of transition metal-catalysed enantioconvergent radical C(sp3)–C coupling with racemic alkyl halides. (b) Enantioconvergent C(sp3)–C(sp) coupling of secondary alkyl halides with Cu(I)/N,N,P-ligand catalysts. (c) Different radical coupling mechanisms for secondary and tertiary alkyl radicals. (d) Rational design of chiral ligands for the enantiocontrol of coupling with tertiary alkyl radicals. (e) Enantioconvergent radical C(sp3)–C(sp) coupling of racemic tertiary alkyl halides with terminal alkynes.
Fu-Li Wang, Research Associate Professor of the Department of Chemistry at SUSTech, Dr. Chang-Jiang Yang, Research Scholar of the Department of Chemistry at SUSTech, and Ji-Ren Liu, Ph.D. student of the Center of Chemistry for Frontier Technologies at Zhejiang University, are the co-first authors of the paper.
Xin-Yuan Liu, Professor of the Department of Chemistry and the Shenzhen Grubbs Institute at SUSTech, Qiang-Shuai Gu, Research Associate Professor of the Academy for Advanced Interdisciplinary Sciences at SUSTech, and Xin Hong, Professor of the Center of Chemistry for Frontier Technologies at Zhejiang University, are the corresponding authors.
Other authors from SUSTech included Ning-Yuan Yang, Xiao-Yang Dong, Ruo-Qi Jiang, Xiao-Yong Chang, Zhong-Liang Li, Dai-Lei Yuan, and Yu-Shuai Zhang, as well as Guo-Xiong Xu from Zhejiang University.
This work was supported by the National Natural Science Foundation of China (NSFC), Guangdong Innovative Program, Shenzhen Special Funds, and the SUSTech Special Fund for the Construction of High-Level Universities.
Paper link: https://doi.org/10.1038/s41557-022-00954-9
To read all stories about SUSTech science, subscribe to the monthly SUSTech Newsletter.
Proofread ByAdrian Cremin, Yingying XIA
Photo By