Pannexins (Panx) are large-pore nonselective membrane channels that mediate substrate exchange between adjacent cells or between cells and extracellular matrix. One of the Panx members, Panx2, can transport small molecules such as ATP and play an important role in cell communication and cell homeostasis. Dysfunction of Panx2 can lead to various diseases, such as ischemic brain injury, glioma, and glioblastoma multiforme. However, the working mechanism of Panx2 is still not clear. The structural study of Panx2 can provide novel insights into the working mechanism of the Panx family.
Research Assistant Professor Huawei Zhang and researchers of the Department of Biomedical Engineering at the Southern University of Science and Technology (SUSTech) recently reported the cryo-EM structure and its working mechanism of Panx2.
Their study, entitled “Cryo-EM structure of human heptameric pannexin 2 channel,” has been published in Nature Communications, a multidisciplinary journal covering the natural sciences, including physics, chemistry, earth sciences, medicine, and biology.
In this work, the researchers screened a series of expression and purification conditions to obtain Panx2 samples suitable for cryo-EM research. Further screening of freezing conditions and data collection were performed in the Cryo-EM Center of SUSTech. The structure of the Panx2 channel was determined at a resolution of 3.4 Å (Fig. 1). Panx2 is composed as a heptamer in a C7-symmetry manner. Each subunit can be divided into three domains: extracellular domain (ECD), transmembrane domain (TMD), and intracellular domain (ICD). Among them, the Q91, E102, S264, and Y94 residues in the ECD interact with the Y82, Q285, Y82, and S278 residues from the adjacent subunits. The CH1 and CH3 helices in the ICD interact with the CH6 and CH7 helices from the adjacent subunits. Together, those sites form the structural basis of Panx2 heptamer.
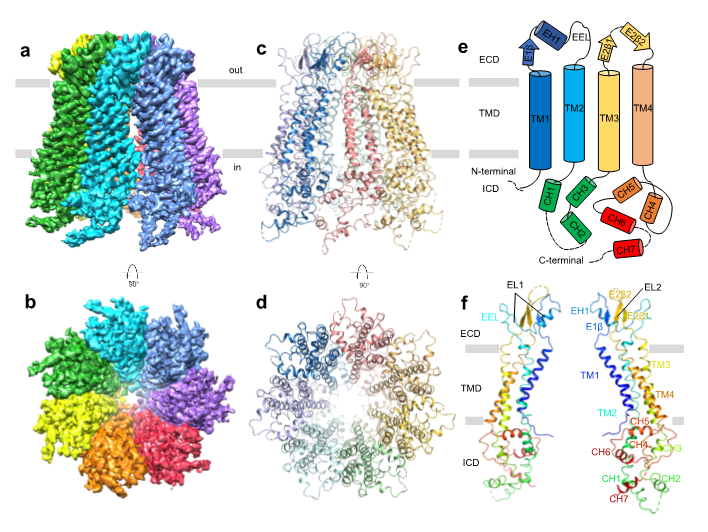
Figure 1. Cryo-EM structure of Panx2
Through structural comparison between Panx2 and Panx1, radius distribution analysis of the Panx2 channel, and the electrostatic distribution analysis of the Panx2 channel, the researchers speculate that the structure of Panx2 obtained in this work adopts an open state. A ring formed by seven R89 arginine residues located at the extracellular entrance forms the narrowest part of the channel and acts as a molecular filter that regulates the opening and closing of the Panx2 channel. These observations were further verified by molecular dynamics simulations and ATP release assays (Fig. 2).
This study deepens our understanding of the Panx2 channel and provides novel insights into the working mechanism of the Panx family.
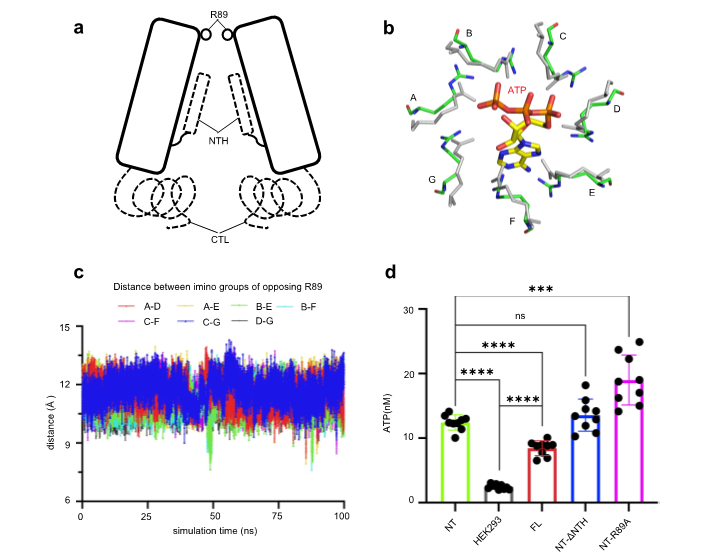
Figure 2. R89 regulates the opening and closing of the Panx2 channel
Dr. Hang Zhang, a postdoctoral fellow from the Department of Biomedical Engineering at SUSTech, is the first author of this paper. Research Asst. Prof. Huawei Zhang from SUSTech and Prof. Shuguang Yuan from the Shenzhen Institutes of Advanced Technology (SIAT) of the Chinese Academy of Sciences (CAS) are the corresponding authors. SUSTech is the first and corresponding affiliation of this paper.
This work was supported by the National Natural Science Foundation of China (NSFC), Science, Technology and Innovation Commission of Shenzhen Municipality, and the Cryo-EM Center of SUSTech.
Paper link: https://www.nature.com/articles/s41467-023-36861-x
To read all stories about SUSTech science, subscribe to the monthly SUSTech Newsletter.
Proofread ByAdrian Cremin, Yingying XIA
Photo By