The research team led by Professor Lei XI from the Department of Biomedical Engineering at the Southern University of Science and Technology (SUSTech) has successfully developed a cortex-wide multimodal microscope. This system enables simultaneous, high-resolution imaging of neural activity and hemodynamics across the entire cerebral cortex of awake mice. Integrating Ca²⁺ imaging, photoacoustic microscopy, and laser speckle contrast imaging, the technology clearly visualizes the dynamic coupling process between neural activity and hemodynamics at single-vessel resolution. The related findings have been published in Nature Communications under the title “A cortex-wide multimodal microscope for simultaneous Ca²⁺ and hemodynamic imaging in awake mice.”
The precise coordination between neural and vascular systems, known as neurovascular coupling, is key to understanding brain function and neurological disorders. Existing imaging techniques are either limited to local fields of view or unable to simultaneously capture neural activity and vascular dynamics, making it difficult to reveal their coupling mechanisms at the whole-cortex scale.
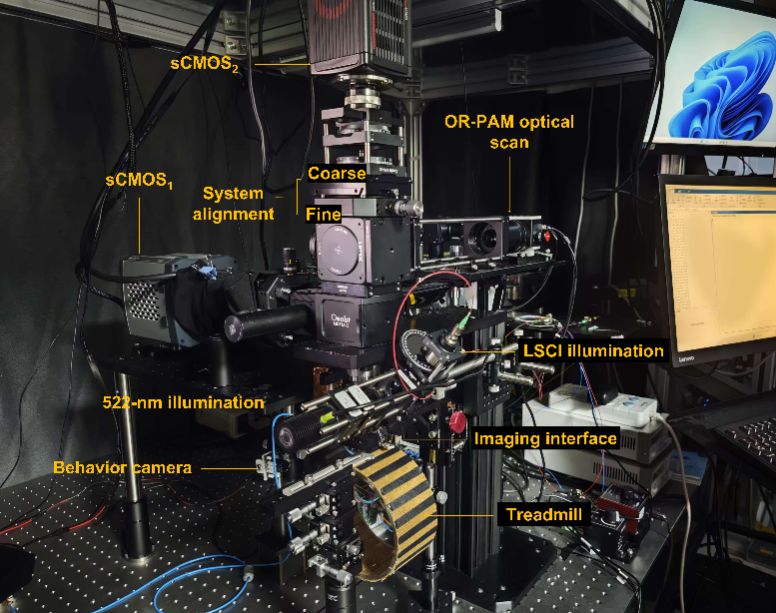
Fig. 1 The multiScope multimodal imaging system enables simultaneous in vivo imaging of neural activity and hemodynamics in the mouse cerebral cortex.
By combining an infinity-corrected rotary scanning mechanism with a composite imaging strategy, the research team successfully integrated three imaging modalities into a single platform (multiScope). The imaging system offers a field-of-view with a diameter of 8.6 mm, a spatial resolution as high as 5.8μm, and an imaging speed of up to four frames per second (FPS). Without the need for exogenous contrast agents, this technology can simultaneously observe neural activity, cerebral blood volume/total hemoglobin, and cerebral blood flow velocity, achieving dynamic imaging across multiple scales and parameters from the cortex to a single vessel. The team successfully observed rapid changes in whole-cortex neurovascular coupling during anesthesia induction and recovery in mouse models. Experiments revealed that the correlation between high-frequency neural signals and blood flow signals significantly decreased under anesthesia and re-strengthened during the awakening process, intuitively demonstrating the “decoupling” and “re-coupling” effects of anesthesia on neurovascular coupling.
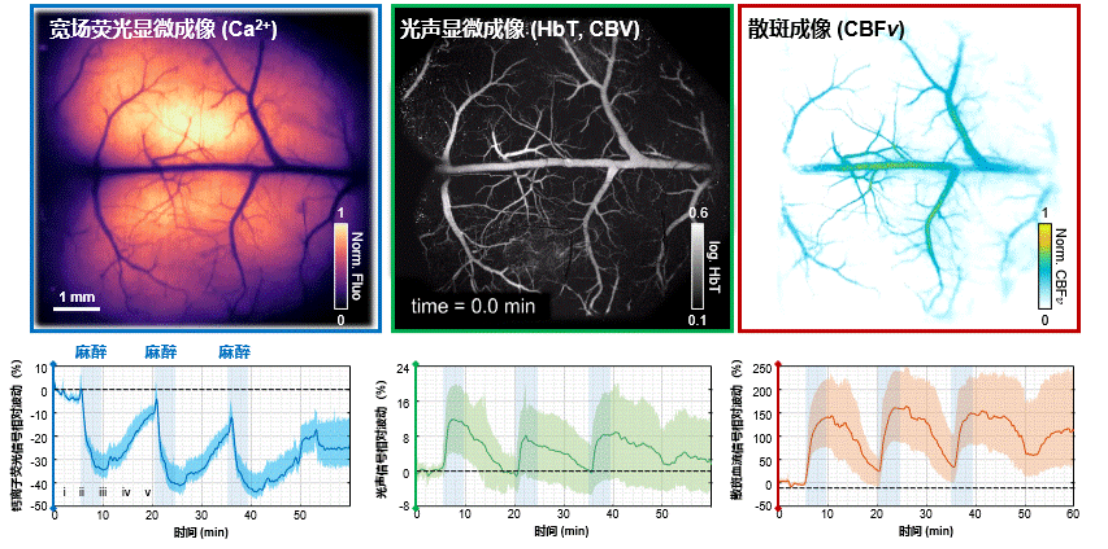
Fig. 2 Neural activity and hemodynamic responses during the awake-anesthesia-recovery process in mice.
In an electroshock-induced epilepsy model, multiScope successfully captured dynamic responses of blood flow and total hemoglobin at the single-vessel level following seizure onset. It was also found that adjacent blood vessels may exhibit opposite regulatory patterns, highlighting the unique advantage of its high spatiotemporal resolution. Furthermore, multiScope integrates three complementary methods for measuring blood flow velocity to meet different research needs: laser speckle contrast imaging can quickly obtain relative blood flow maps across the entire cortex; photoacoustic decorrelation analysis enables label-free absolute blood flow velocity measurement; and fluorescence localization microscopy based on fluorescent contrast agents can provide super-resolution blood flow tracking beyond the diffraction limit.
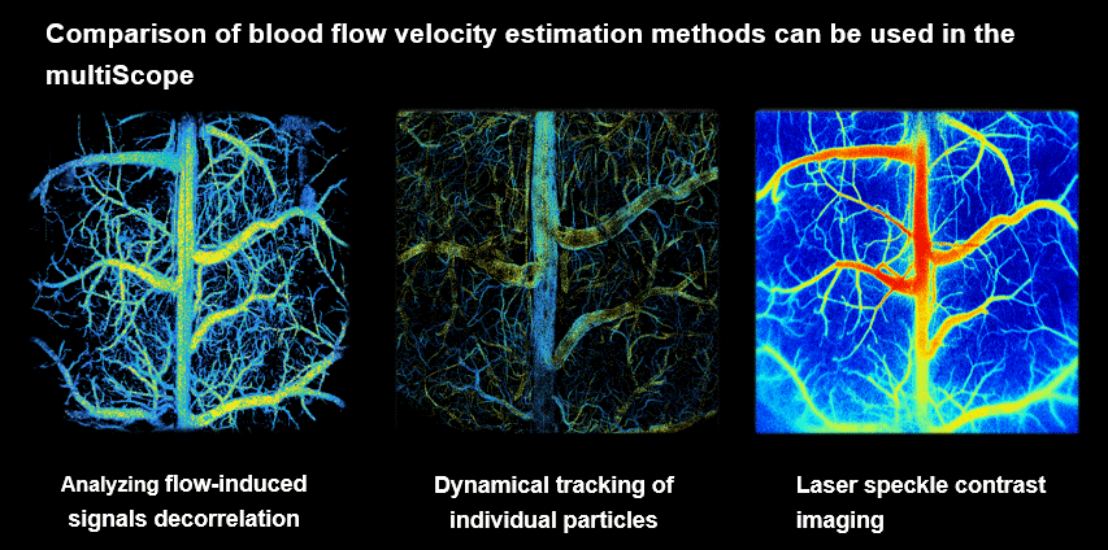
Fig. 3 Comparison of blood flow imaging methods in the mouse cerebral cortex using multiScope.
This research not only provides a powerful observation tool for neuroscience but also offers a new technological pathway for understanding the neurovascular mechanisms of diseases such as Alzheimer’s and epilepsy. In the future, by combining objectives with higher numerical apertures and novel genetically encoded probes, multiScope is expected to achieve whole-brain functional imaging at the capillary level, driving a paradigm shift in brain science and brain disease research.
SUSTech is the corresponding affiliation of the paper. Wei QIN, Tingting LI, and Linyang LI from the Department of Biomedical Engineering are co-first authors, and Professor Lei XI is the corresponding author.
Link: https://doi.org/10.1038/s41467-025-64393-z
Proofread ByNoah Crockett, Yifei REN
Photo ByWei QIN