Gammaherpesviruses are one of the three major subfamilies of the Herpesviridae and widely infect a broad range of mammals, including humans, causing significant disease. This subfamily includes two important human oncogenic viruses, Epstein-Barr virus (EBV) and Kaposi’s sarcoma–associated herpesvirus (KSHV), both of which are closely linked to multiple malignancies and autoimmune diseases. Coinfection with EBV and KSHV is associated with particularly severe tumors, such as primary effusion lymphoma (PEL). In addition, animal gammaherpesviruses infecting livestock such as sheep and cattle cause devastating diseases characterized by malignant fever and infertility. Despite their medical and veterinary significance, effective vaccines and neutralizing antibodies against gammaherpesviruses are still lacking. Achieving cross-protection across different viral genera and host species has long remained a central challenge in the field.

As enveloped viruses, gammaherpesviruses rely on a highly conserved membrane fusion process to enter host cells. Glycoprotein B (gB) is an essential fusion protein shared by all herpesviruses and is considered a promising target for broad-spectrum intervention. However, previous studies have largely focused on human gammaherpesviruses, leaving the structural and functional understanding of animal gammaherpesvirus gB incomplete. Whether a common neutralization vulnerability exists across genera and species has remained unclear, hindering the development of gB-based broadly protective vaccines and antibodies.
To address this critical question, the team led by Chinese Academician Mu-Sheng ZENG and Cong SUN at the Sun Yat-sen University Cancer Center collaborated with Zheng LIU’s team at the Cryo-Electron Microscopy Center and Department of Pharmacology, School of Medicine, SUSTech. Leveraging a previously developed EBV chimeric nanoparticle vaccine (Advanced Materials), researchers employed mouse immunization followed by antigen-specific single B-cell screening and sequencing, and identified a gB-targeting monoclonal antibody, Fab5. This antibody was found for the first time to exhibit cross-genus gB binding and broad neutralizing activity against gammaherpesviruses. By determining high-resolution structures of Fab5 in complex with gB from multiple gammaherpesviruses, the study identified a conserved neutralization epitope shared across gammaherpesvirus genera. The study, entitled “A broadly protective antibody targeting gammaherpesvirus gB,” was published online as an accelerated preview in Nature on February 2, 2026. This work represents the first report of a neutralizing monoclonal antibody with broad-spectrum anti-gammaherpesvirus activity and reveals a conserved, functionally critical neutralization site on gB, providing a structural blueprint for rational antibody and vaccine design.

The researchers selected gB proteins from a range of human and animal gammaherpesviruses to systematically assess the cross-genus recognition capacity of Fab5. Biolayer interferometry and cell-surface binding assays showed that Fab5 efficiently binds gB from EBV, rhesus lymphocryptovirus (rhLCV), and murine gammaherpesvirus 68 (MHV68), with substantially broader binding breadth than previously reported gB-neutralizing antibodies. Functional cell-cell fusion assays further demonstrated that Fab5 broadly inhibits gB-mediated membrane fusion across multiple gammaherpesviruses, indicating strong functional conservation of its target epitope. In authentic virus neutralization assays, Fab5 effectively blocked infection by EBV, KSHV, rhLCV, and MHV68 in multiple cell types.
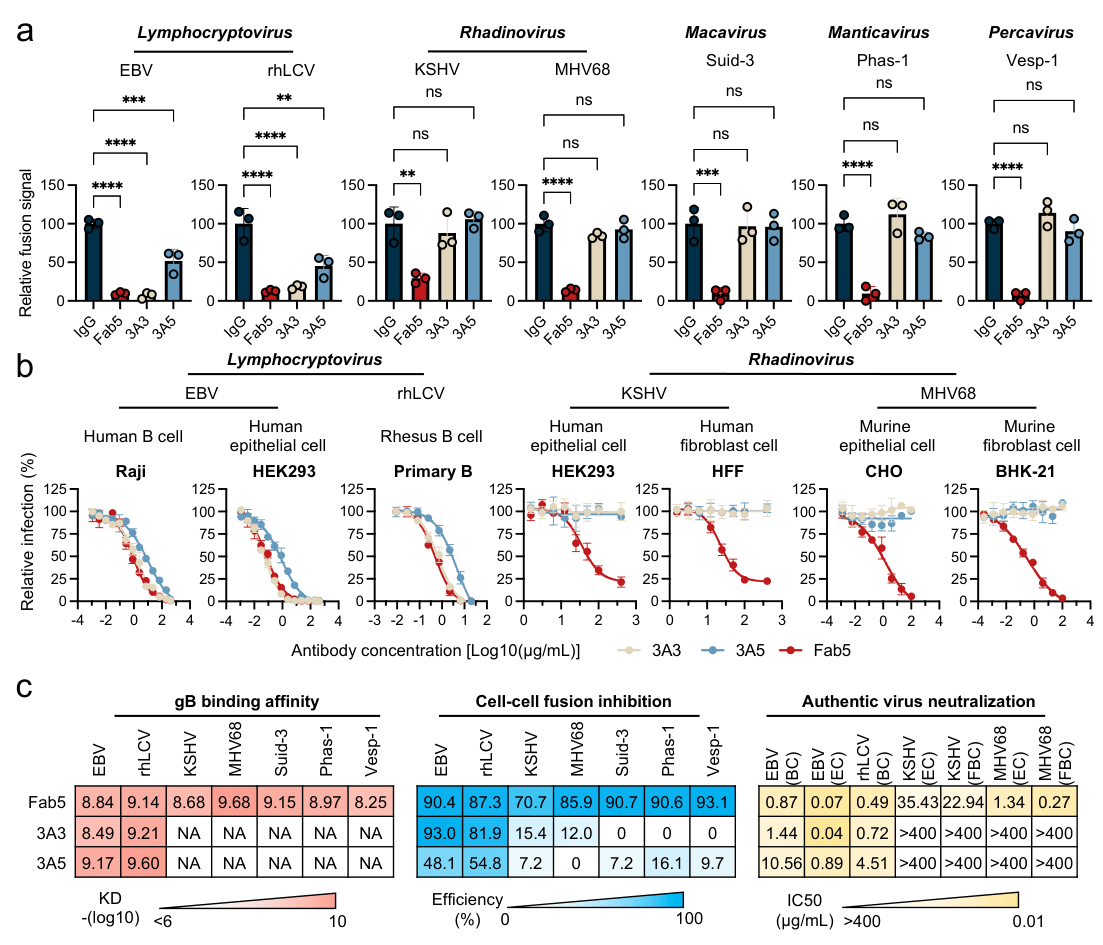
Figure 1. Fab5 broadly inhibits gammaherpesvirus membrane fusion and viral infection.
Based on these in vitro findings, researchers systematically evaluated the protective efficacy of Fab5 in multiple animal models. In a humanized mouse model coinfected with EBV and KSHV, passive immunization with Fab5 significantly improved survival and markedly reduced viral loads of both viruses in the spleen and other tissues. Histopathological and in situ hybridization analyses showed that Fab5 suppressed virus-associated lymphoproliferation and pathological changes, whereas a control antibody targeting only EBV failed to ameliorate KSHV-associated pathology.
In animal gammaherpesvirus models, infection and latency were established using MHV68 in immunocompetent mice. Prophylactic administration of Fab5 significantly reduced MHV68-induced splenomegaly, lowered tissue viral copy numbers, and decreased the number of latently infected cells. Therapeutic administration also conferred partial viral suppression. Moreover, in a rhesus macaque model susceptible to rhLCV, passive immunization with Fab5 nearly completely blocked viremia and oral viral shedding, with no evident adverse effects or seroconversion observed.
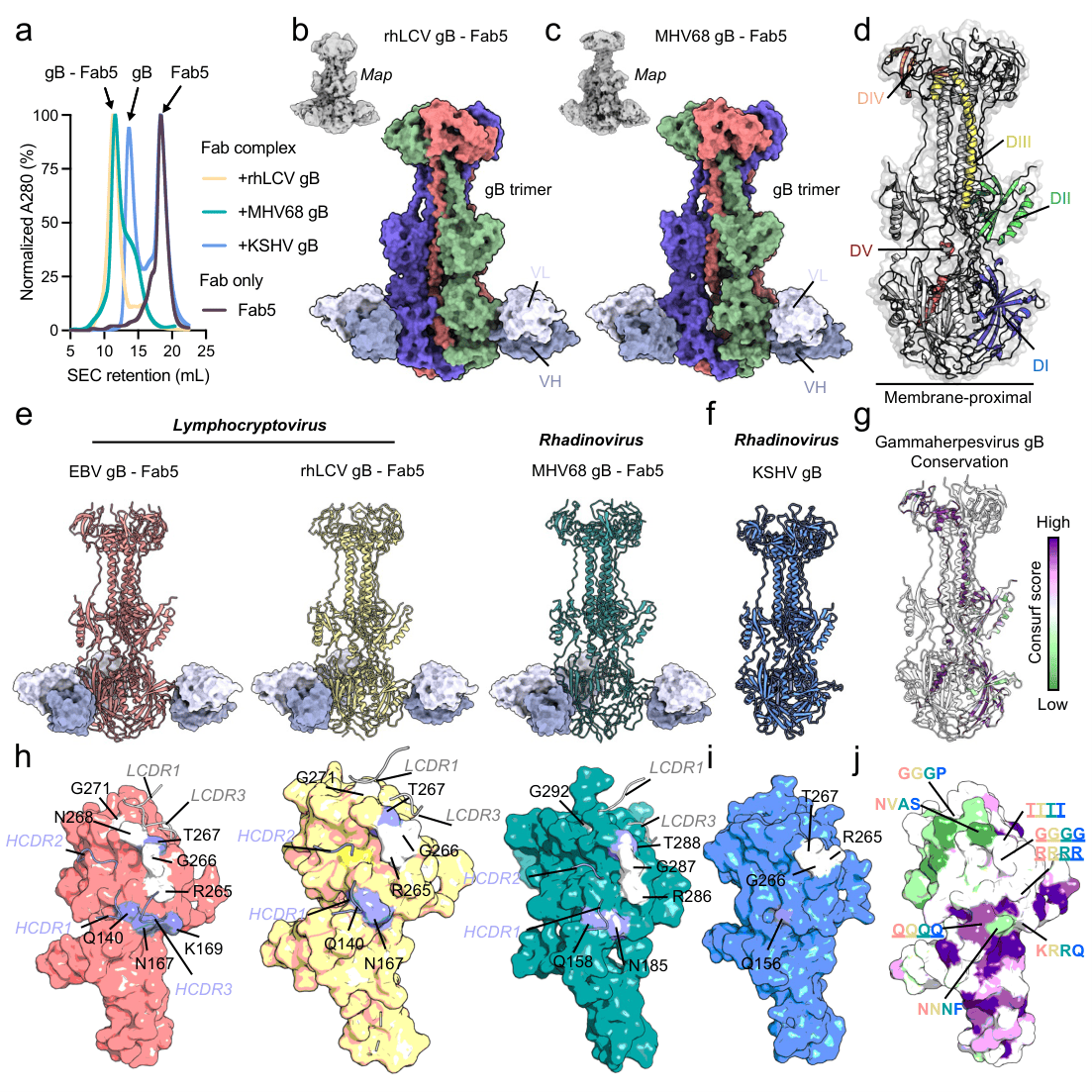
Figure 2. Fab5 recognizes a conserved neutralizing epitope on gammaherpesvirus gB.
To elucidate the molecular mechanism underlying Fab5’s broad neutralizing activity, the researchers solved cryo-electron microscopy structures of Fab5 in complex with representative gammaherpesvirus gB proteins from EBV, rhLCV, and MHV68. The structures revealed that Fab5 binds to Domain I (DI) of gB, a region that is highly conserved in overall architecture across gammaherpesvirus. Detailed structural analysis showed that Fab5 engages a cluster of highly conserved residues within DI through coordinated interactions involving both heavy and light chains, forming an interaction network dominated by hydrogen bonds and side-chain contacts.
Comparative analyses revealed that although KSHV gB shares a highly similar overall conformation with other gammaherpesvirus gBs, it contains fewer conserved residues in DI that are compatible with Fab5 binding, providing a structural explanation for Fab5’s relatively weaker binding and neutralization of KSHV. Sequence and structural mapping further demonstrated that the Fab5-recognized DI epitope exhibits functional conservation despite partial sequence variability across genera. This domain remains structurally stable and surface-accessible in both pre-fusion and post-fusion conformations of gB, defining a conserved neutralization epitope. These findings directly link broad gammaherpesvirus neutralization to a well-defined, cross-genus conserved structural site, offering concrete guidance for rational gB-based vaccine and antibody design.
In summary, this study identified a broadly neutralizing antibody, Fab5, that provides cross-protection against gammaherpesviruses in multiple host and viral models, offering a promising therapeutic prototype for combating herpesviruses, particularly oncogenic herpesviruses. Through high-resolution structural characterization, this study defines a conserved, functionally critical neutralization epitope on gB, laying a theoretical foundation for immunogen optimization, induction of Fab5-like antibody responses, and the rational development of broad-spectrum herpesvirus vaccines and antibody therapeutics.
Chinese Academician Mu-Sheng ZENG and Associate Researcher Cong SUN of the Sun Yat-sen University Cancer Center, together with Professor Zheng LIU of the Cryo-Electron Microscopy Center and Department of Pharmacology, School of Medicine, Southern University of Science and Technology, are the co-corresponding authors of this study. Associate Researcher Cong SUN, postdoctoral fellow Chu XIE (Sun Yat-sen University Cancer Center), and master’s program graduate student Bing-Zhen CHENG (SUSTech) are co-first authors.
Paper Link: https://www.nature.com/articles/s41586-026-10192-5
Proofread ByNoah Crockett, Junxi KE
Photo By