The brain functions as a precisely regulated “super-network,” where tens of billions of neurons serve as fundamental “nodes” with synapses as functional “junctions” that link these nodes to mediate signal transmission. Neurotransmitters, the “signal messengers” released from presynaptic terminals, are recognized by the receptors on postsynaptic membranes, thereby facilitating the transduction of excitatory or inhibitory signals and maintaining brain functions. During synaptic development, synaptic membrane-associated cell adhesion molecules (CAMs) and secretory proteins within the synaptic cleft act as “molecular bridges” to connect pre- and postsynaptic compartments, which is essential for synapse formation and brain development.
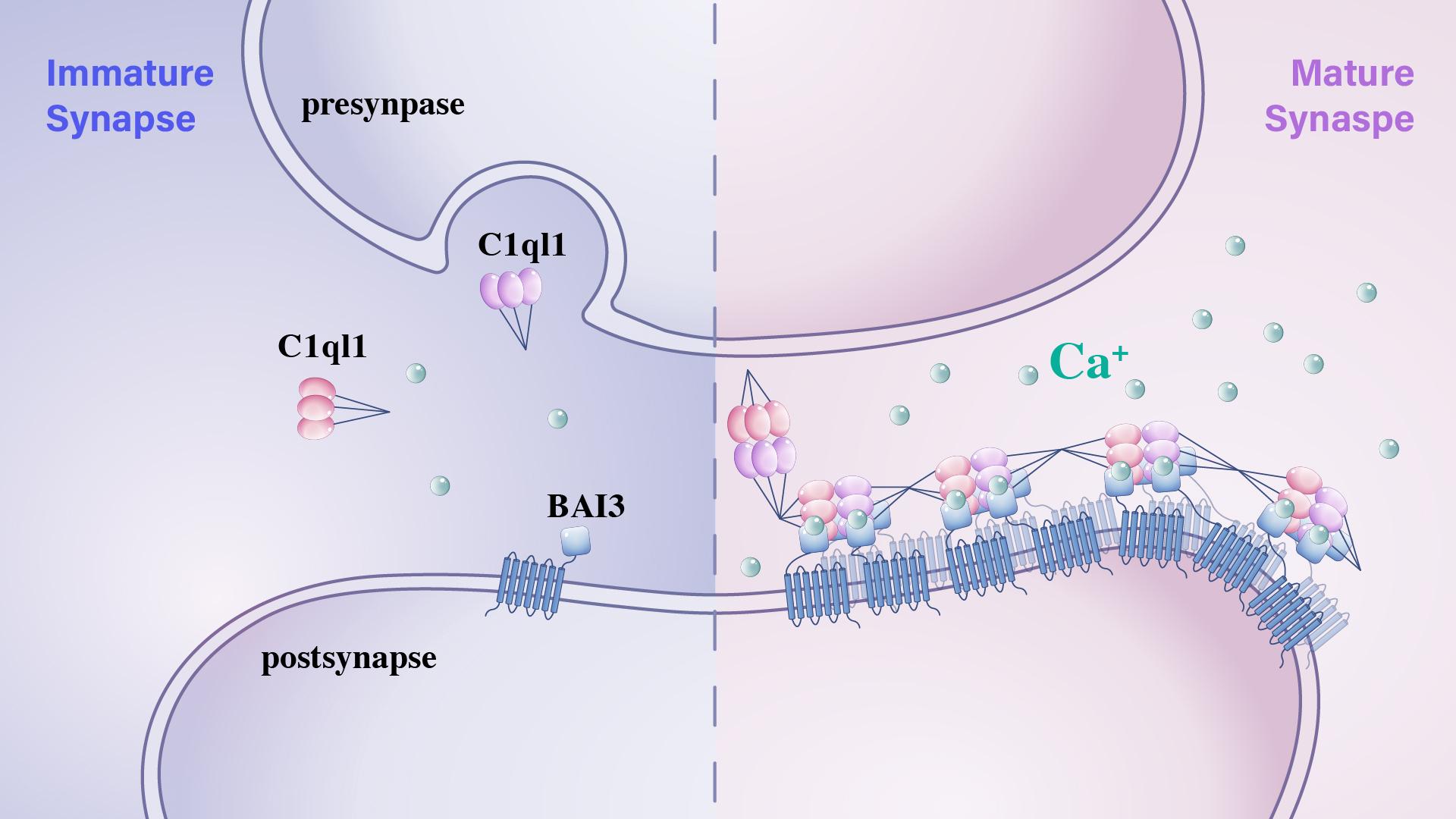
The C1ql family, a class of neurosecretory proteins, has been identified as a key mediator of synaptic connectivity in multiple brain regions. Specifically, the interaction between C1ql1 and the postsynaptic adhesion G protein-coupled receptor (GPCR) BAI3 is critical for the maintenance of synaptic connections between climbing fibers (CFs) from the inferior olive nucleus and Purkinje cells (PCs) in the cerebellum. Mutations in either C1ql1 or BAI3 have been reported to impair brain functions related to sensory perception and motor learning. However, the molecular mechanisms underlying the assembly of C1ql1/BAI3 synaptic adhesion complexes remain elusive.
The research group, led by Dr. Zhiyi WEI from the School of Life Sciences at Southern University of Science and Technology (SUSTech), in collaboration with Dr. Bo ZHANG’s group at Peking University Shenzhen Graduate School and Shenzhen Bay Laboratory, published a research article entitled “Structural basis of calcium-dependent C1ql1/BAI3 assemblies in synaptic connectivity” in Nature Communications. This study resolved the high-resolution structures of the C1ql1 hexamer and its complex with BAI3, thereby elucidating the calcium-modulated mechanisms of C1ql1 oligomerization and C1ql1/BAI3 binding that regulate synapse development and maturation.
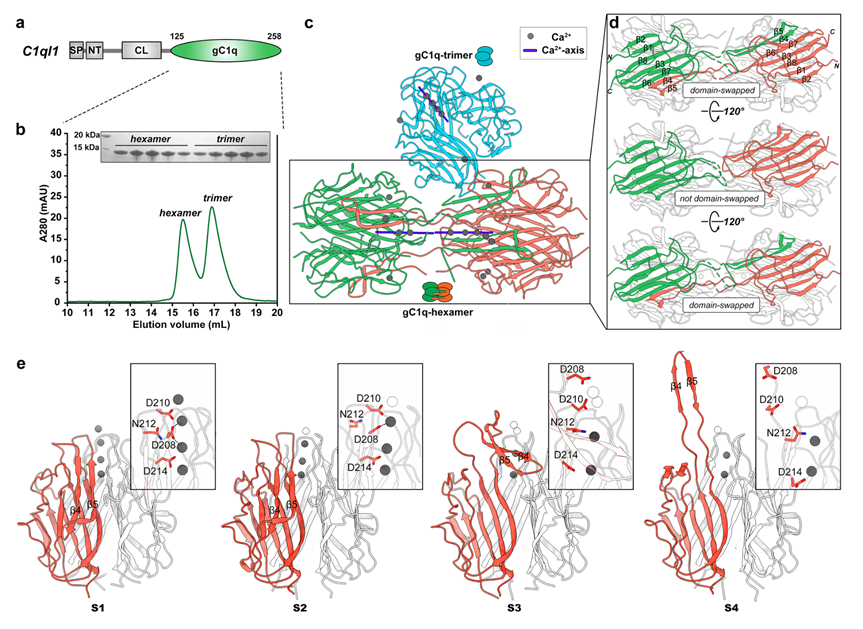
Figure 1. C1ql1-gC1q crystal structure and MD analyses uncover its hexamer/trimer transition in a calcium-dependent manner
The researchers first discovered that the gC1q domain of C1ql1 can assemble into a hexameric form, in addition to its well-characterized trimeric form. Crystallographic analysis of the C1ql1-gC1q hexamer revealed that this hexamer is formed by domain-swapping between two trimers. Combining with structural, biochemical, and molecular dynamics (MD) simulation analyses, the researchers further demonstrated that calcium ions, which localize at the central axis of the gC1q trimer, serve as a key regulatory switch that drives the trimer-hexamer transition of C1ql1-gC1q (Figure 1).
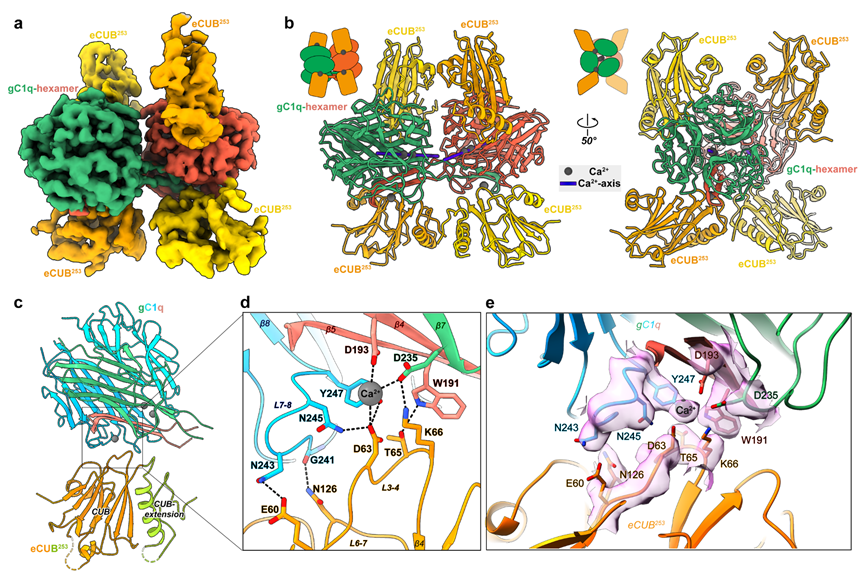
Figure 2. Cryo-EM structure of C1ql1-gC1q and BAI3-eCUB complex
To dissect the C1ql1/BAI3 interaction mechanism, the researchers also determined the cryo-electron microscopy (cryo-EM) structure of the complex containing the extended CUB domain of BAI3 (BAI3-eCUB) and the C1ql1-gC1q hexamer using single-particle analysis. Notably, calcium ions also mediate the C1ql1-gC1q/BAI3-eCUB interaction by localizing at their interface to coordinate them together (Figure 2). By a combination of structural, biochemical, cellular experiments, and mouse experiments, the researchers verified that the trimer/hexamer transition of C1ql1-gC1q is essential for the assembly of synaptic adhesion complexes. Disruption of this transition leads to defects in CF-PC synapse development.
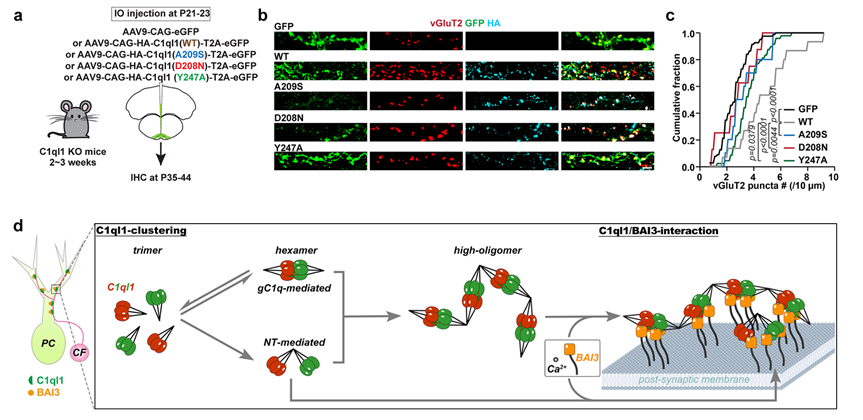
Figure 3. C1ql1 clustering enhances the C1ql1 and BAI3 assemblies, promoting the CF-PC synapse development
The researchers recombinantly expressed and purified the full-length C1ql1 protein, and negative stain electron microscopy (ns-EM) analysis revealed that, in addition to forming C-terminal gC1q hexamers, C1ql1 can also establish the N-terminal intermolecular linkages via two conserved cysteine residues. Both “tail-to-tail” and “head-to-head” oligomerization modes drive full-length C1ql1 to assemble into higher-order linear clusters, which serve as an “assembly platform” in the synaptic cleft. Importantly, this C1ql1 cluster platform significantly enhances the binding efficiency to postsynaptic BAI3 receptors, thereby promoting the development and maturation of CF-PC synapses (Figure 3).
Co-first authors of this paper are Liangyu LIAO, PhD candidate in the School of Life Sciences at SUSTech; Dr. Ying HAN, School of Chemical Biology and Biotechnology at Peking University Shenzhen Graduate School, and currently a postdoctoral fellow at Shenzhen Bay Laboratory; Dr. Fengfeng NIU, School of Life Sciences at SUSTech; and Dr. Yingjie WANG, Dr. Jiali Gao’s group with the Institute of Systems and Physical Biology at Shenzhen Bay Laboratory. Corresponding authors are Dr. Zhiyi WEI, School of Life Sciences at SUSTech, and Dr. Bo ZHANG, School of Chemical Biology and Biotechnology at Peking University Shenzhen Graduate School and Shenzhen Bay Laboratory. SUSTech is the first completing institution.
Proofread ByNoah Crockett, Junxi KE
Photo ByYan QIU