Assistant Professor Xin HONG at the Department of Biochemistry, SUSTech Homeostatic Medicine Institute, School of Medicine at Southern University of Science and Technology, in collaboration with Attending Physician Dr. Jun TAN from the Department of Neurosurgery at Xiangya School of Medicine, Central South University, Associate Chief Physician Dr. Weinan GUO from Xijing Hospital of Air Force Medical University and Chief Physician Dr. Ling GUO from the Department of Nasopharyngeal Carcinoma at Sun Yat-sen University Cancer Center, published a research letter online in the prestigious international cancer journal Journal of Hematology & Oncology. The paper is titled “CBX3 confers ferroptosis resistance during blood-borne metastasis.”
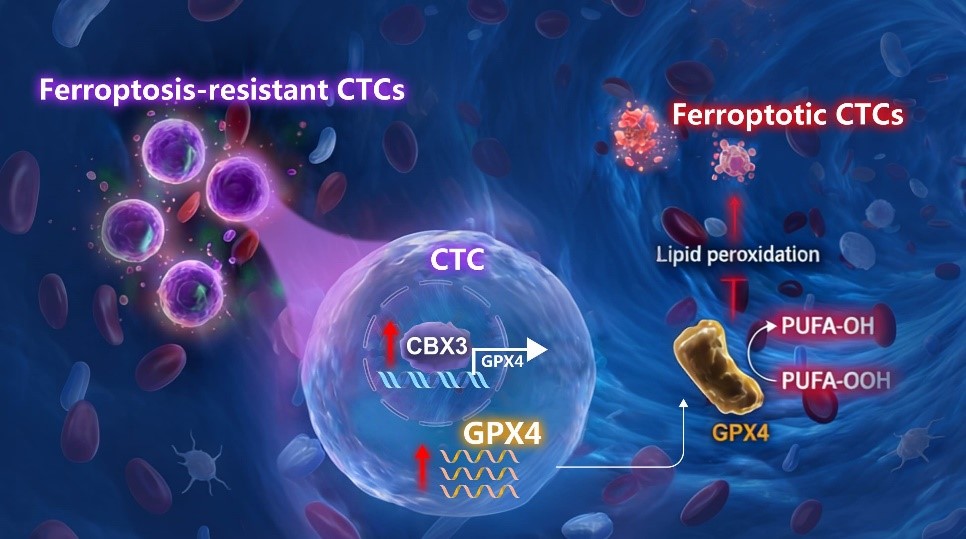
By integrating single-cell transcriptomic analysis with rigorous functional and clinical validation, this study systematically elucidates a critical mechanism governing brain metastasis. We demonstrate that circulating tumor cells (CTCs) evade ferroptosis via a chromobox homolog 3 (CBX3)-mediated regulation of glutathione peroxidase 4 (GPX4) expression. This axis is essential for promoting CTC survival during hematogenous dissemination, thereby facilitating distant metastasis. Crucially, we propose that dynamic monitoring of CBX3+GPX4+ CTCs serves as a potent liquid biopsy biomarker for tracking hematogenous metastasis in patients.
While CTCs represent the “seeds” of metastasis shed from primary tumors into the circulation, the vast majority succumb to environmental stressors, including oxidative stress and immune surveillance, characterized by elevated intracellular reactive oxygen species (ROS) and mitochondrial dysfunction. Given that brain metastasis remains a primary cause of cancer-related mortality, investigating the survival mechanisms of CTCs in this context is vital. To address this, we enrolled four lung cancer patients presenting with brain metastases. We isolated CTCs from whole blood samples and performed paired single-cell RNA sequencing (scRNA-seq) on both the isolated CTCs and brain metastasis tissues (Fig. 1). A total of 1,153 CTCs were recovered across the cohort. The malignancy of these isolated cells was confirmed through multi-omic analysis: mRNA expression levels of established epithelial tumor markers (EPCAM, KRT18, KRT8) (Fig. 1E), and antibody-derived tag (ADT)-based EPCAM protein quantification (Fig. 1F); inference of chromosomal instability (CIN) and copy number variations (CNVs) (Fig. 1H-I); and identification of somatic tumor mutations (Fig. 1K).
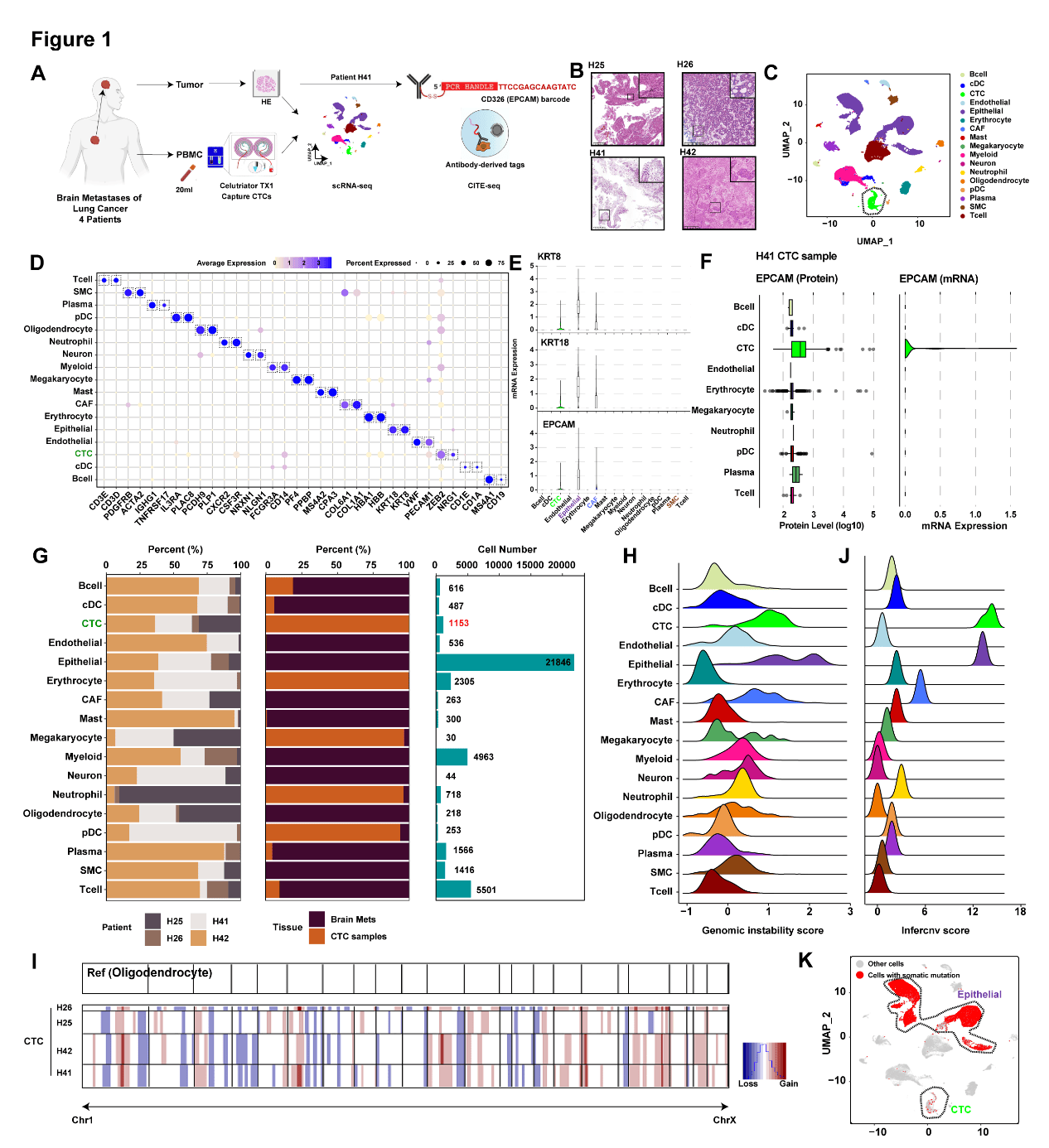
Figure 1 Single-cell omic characterization of CTCs from LUAD patients with BrM
Mechanistically, CTCs predisposed to brain metastasis are characteristically in a ferroptosis-resistant state. These CTCs show significantly upregulated expression of the transcription factor CBX3, which is strongly correlated with the expression of the key anti-ferroptosis factor GPX4. Functionally, CBX3 protects CTCs from ferroptosis by regulating GPX4 expression. In H1975 lung cancer and A375 melanoma cell lines, CBX3 knockdown promotes ferroptosis and significantly reduces tumor cell survival and invasiveness. Conversely, CBX3 overexpression enhances anti-ferroptosis capability, growth, migration, and invasion in vitro, as well as metastasis in vivo (Fig. 2). In lung cancer and melanoma patient cohorts, both the counts of CBX3+GPX4+ CTCs and the proportion of CBX3+GPX4+ CTCs among total CTCs are significantly correlated with tumor metastasis, suggesting that CBX3+GPX4+ CTCs may represent potential non-invasive biomarkers (Fig. 2I).
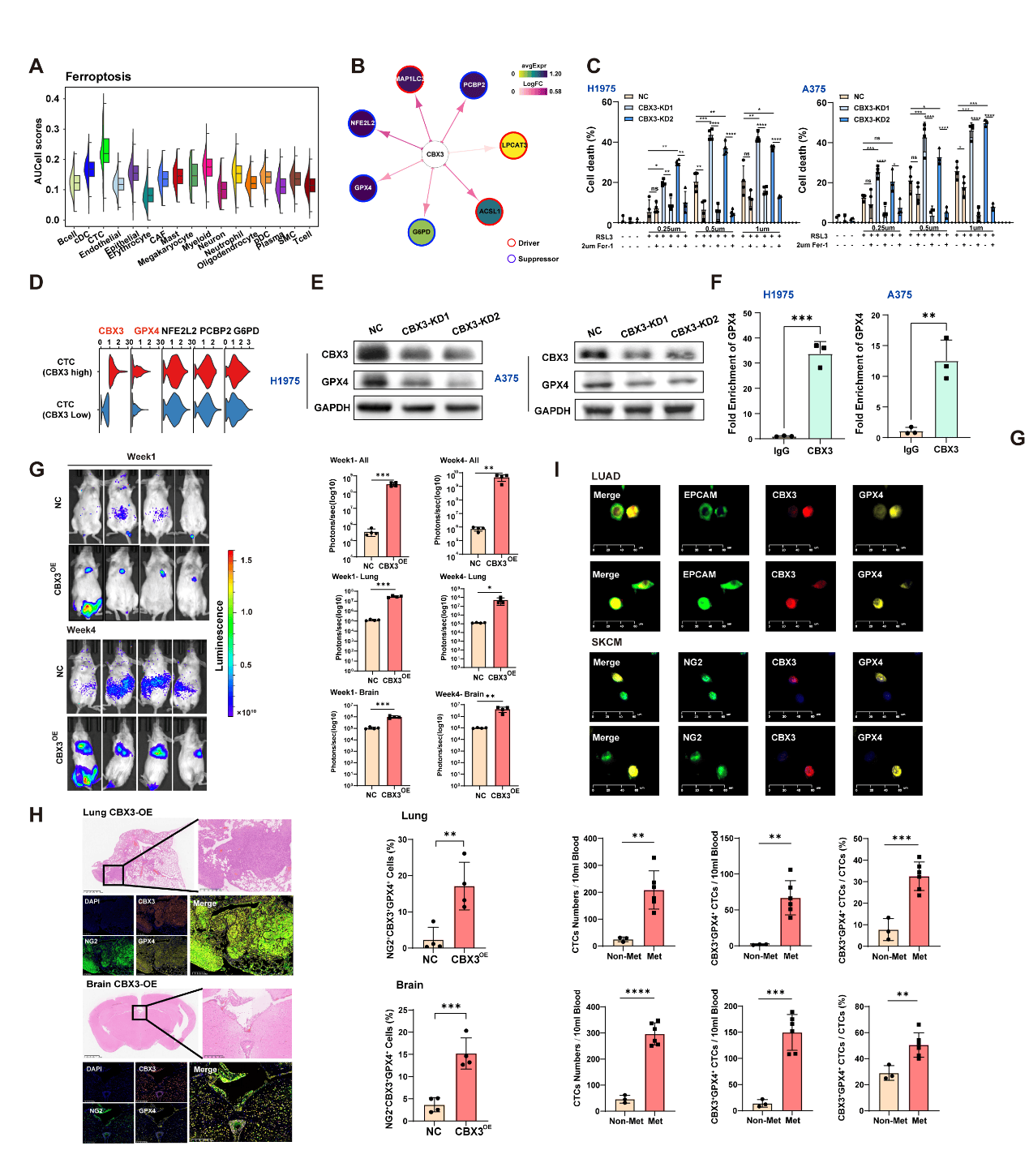
Figure 2 CBX3 drives ferroptosis resistance and tumor progression through direct regulation of GPX4.
Collectively, these findings identify the CBX3-GPX4 axis as a pivotal driver of ferroptosis resistance in CTCs prone to brain metastasis. CBX3 promotes ferroptosis evasion to fuel CTC-mediated metastatic progression. Clinically, real-time monitoring of CBX3+GPX4+ CTCs may enable precise prediction of metastatic progression, underscoring their significant clinical potential as liquid biopsy biomarkers.
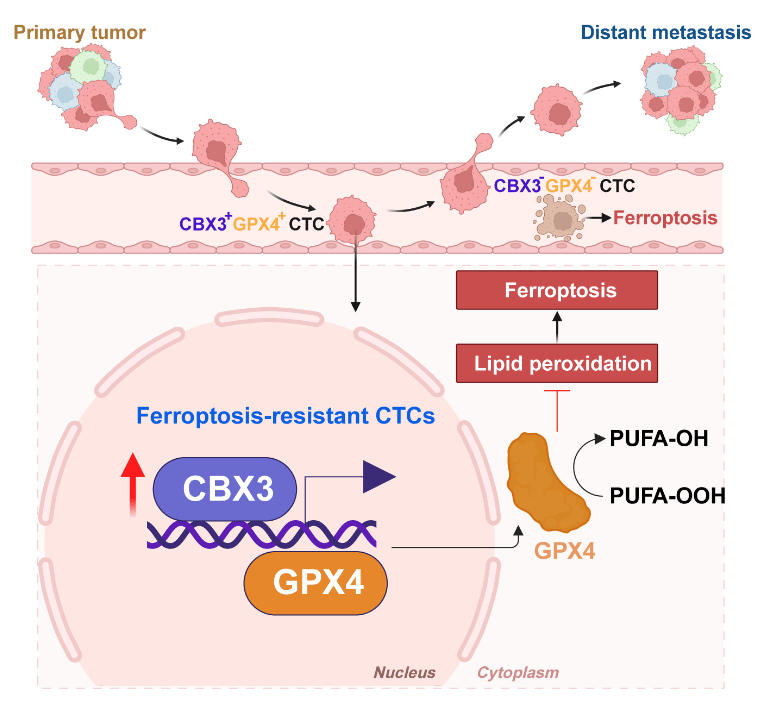
Schematic diagram of the mechanism
The co-first authors of this paper are Chun WU (MD candidate, SYSUCC, and visiting student at SUSTech), Xuefei LIU (PhD candidate, SUSTech) and Boxi ZHAO (Master candidate, SUSTech). The co-corresponding authors are Assistant Professor Xin HONG from the Department of Biochemistry, SUSTech Homeostatic Medicine Institute, and School of Medicine at SUSTech; Attending Physician Jun TAN from Xiangya School of Medicine, Central South University; and Associate Chief Physician Dr. Weinan GUO from Xijing Hospital, Air Force Medical University. SUSTech is the primary institute for the first author and last corresponding author.
Paper Link:https://pubmed.ncbi.nlm.nih.gov/41540451 (DOI: 10.1186/s13045-025-01777-0)
Proofread ByNoah Crockett, Junxi KE
Photo ByYan QIU