Chiral vicinal diamines represent key structural elements in a large number of natural products, pharmaceutical agents, and agrochemicals. They also constitute excellent platforms for the development of chiral ligands, organocatalysts, and auxiliaries with broad utility in asymmetric synthesis. Consequently, expedited assembly of vicinal diamines from readily available precursors has long been recognized as a preeminent goal in organic synthesis. Recently, Professor Liu and co-workers reported the use of O-acylhydroxylamines as dialkylaminyl radical precursors to trigger asymmetric diamination of alkene under Cu(I)/chiral phosphoric acid dual catalysis. This reaction allows for direct alkylamine incorporation and features high enantioselectivity, a broad substrate scope, wide functional-group tolerance, and mild reaction conditions, providing convenient and practical access to a wide range of highly enantio-enriched b-alkylamine-containing pyrrolidines. Noteworthy is the dual role of O-acylhydroxylamine as both an alkylamine source and an oxidant in this process, which would not only circumvent the catalyst poisoning associated with the use of a free alkylamine but also dispense with excess external oxidants required in oxidative diamination reactions, thus overcoming challenges in previous asymmetric diamination of alkenes. This work was published in Chem (Chem 2017, 10.1016/j.chempr.2017.10.008).
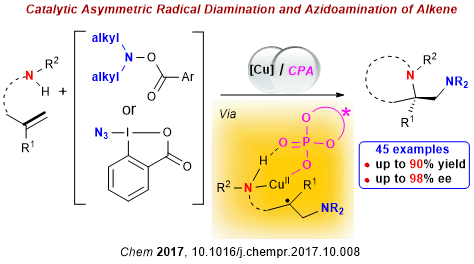
Scheme 1 Asymmetric Radical Diamination of Alkenes
Recently, Liu’s group have successfully developed a catalytic asymmetric radical α-cyclopropanation of alkenyl aldehydes in the presence of a Cu(I)/chiral secondary amine cooperative system, which provides facile access to bicyclo[3.1.0]hexane skeletons bearing two highly congested vicinal all-carbon quaternary stereocenters of significant bioactivity. The simple α-methylene group of an aldehyde, instead of unstable metallocarbene precursors, served as a good C1 source in this efficient asymmetric [2+1] cycloaddition, which constitutes an ideal strategy for cyclopropanation with respect to ready accessibility of starting materials, operation safety and atom-economy. The use of copper iodide, cyclic hypervalent iodine(III) reagent as the single electron oxidant and bulky chiral secondary amine play an important role in the context of this transformation. Meanwhile, the reaction exhibited a remarkably broad substrate scope covering diverse aromatic, heteroaromatic, alkenyl, alkyl-substituted geminal alkenes with excellent functional group tolerance. This work has been accepted for publication on Nature Communication (in press).
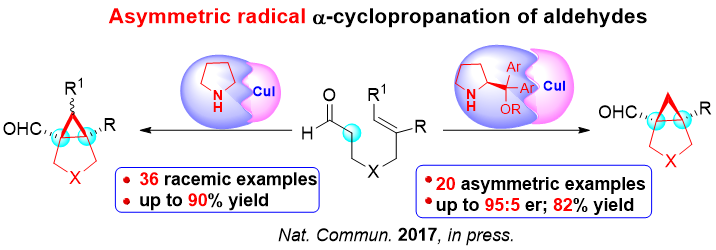
Scheme 2 Asymmetric Radical α-Cyclopropnation of Alkenyl Aldehydes
Medium-sized and medium-bridged cyclic alkenes are attractive structural motifs in natural products and therapeutic agents. Due to the unfavorable entropic and/or enthalpic factors with these ring systems, their efficient construction remains a formidable challenge. A revolutionary advancement in this field is the establishment of transition metal-catalyzed ring-closing metathesis. However, high-dilution and/or slow-addition operation has been frequently required to inhibit the undesired competitive oligomerization via intermolecular olefin metathesis. To circumvent these problem, Professor Liu’ group recently reported, for the first time, a novel C–C bond reorganization strategy via an unprecedented radical 1,3-, 1,4-, or 1,5-vinyl migration triggered by various types of fluoroalkylation of alkenes for the efficient realization of 1,2-fluoroalkylalkenylation reaction. This strategy provided a novel and operationally simple alternative method to construct the synthetically challenging medium- and large-sized cyclic alkenes. An array of 8-14-membered medium-/large-sized cyclic alkenes are constructed with multiple functional group tolerance. This work was published in Science Advances (Sci. Adv. 2017, 3, e1701487).
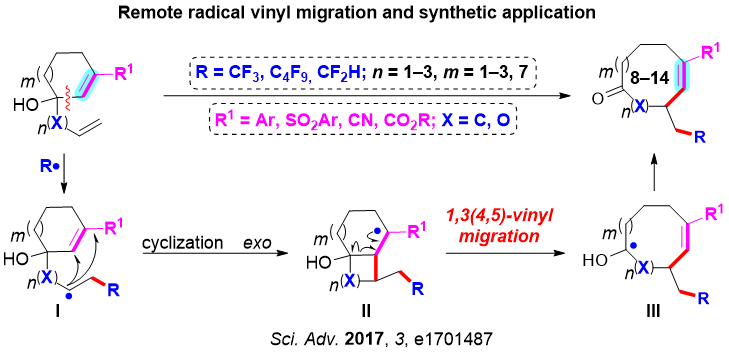
Scheme 3 Remote Radical Vinyl Migration for Synthesis of Cyclic Alkenes
Since joining SUSTech, Professor Xin-Yuan Liu and his co-workers have published 43 papers in high-level international journals, including 17 top-level journals: Chem (1), Sci. Adv. (1), Nat. Commun. (4), JACS (3) and ACIE (8), etc. All the work has won support from National Natural Science Foundation of China, National Key Basic Research Program of China (973 Program), Shenzhen Scientific Innovation Commission and especially from the SUSTech President Foundation. In particular, he thanked SUSTech President Shiyi Chen for the support from the university.
Related links:
http://www.cell.com/chem/fulltext/S2451-9294(17)30436-9
http://advances.sciencemag.org/content/advances/3/11/e1701487
Liu Xin Yuan Research Group Website: http://liuxy.chem.sustc.edu.cn
Proofread By
Photo By