The Myosin superfamily proteins function as molecular motors in various cellular functions, including muscle contraction, material transport, and force sensing. Compared to other myosins, Myosin VI moves in the opposite direction along the actin filaments within cells, making it unique yet vital for maintaining the balance of intracellular material circulation.
Dysfunction of Myosin VI is often associated with severe conditions such as deafness and cardiomyopathy. Structurally, Myosin VI consists of several distinct regions: the N-terminal motor domain with ATPase activity powers its movement along actin filaments, the central lever arm region bound to calmodulin (CaM) determines the step size on walking, and the C-terminal tail domain serves to bind various cargoes (Figure 1).
In the absence of cargo, Myosin VI adopts an autoinhibited conformation, which is released upon receiving cargo-activation signals, transitioning into an active state to initiate cargo transport. Despite extensive structural study on individual domains of Myosin VI over the past two decades, the understanding of its overall structure and the ‘on-off’ mechanism remains limited.
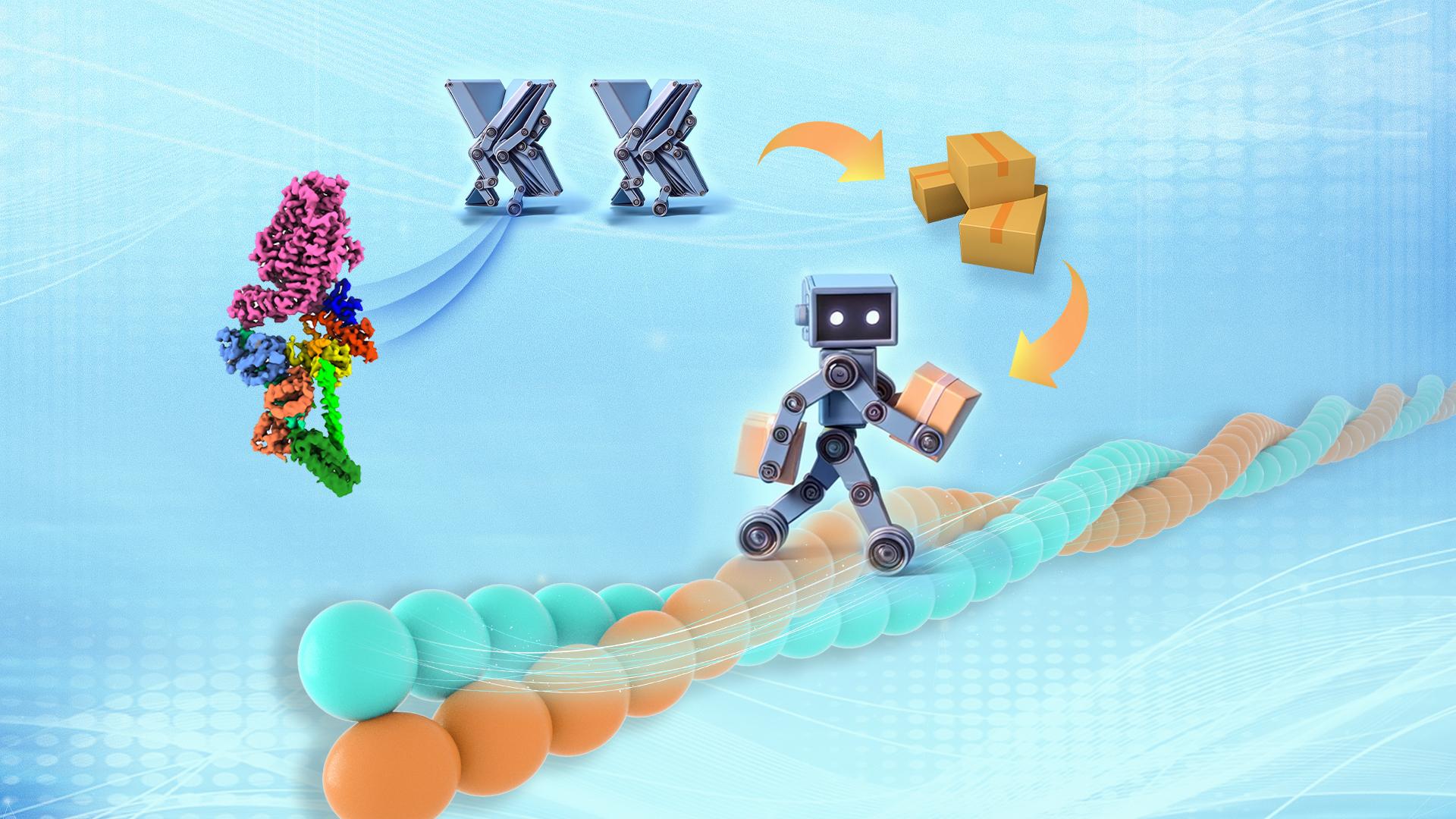
A groundbreaking study published by a team led by Associate Professor Zhiyi Wei from the Department of Neuroscience and Associate Professor Cong Yu from the Department of Chemical Biology at the Southern University of Science and Technology (SUSTech) has determined the high-resolution cryo-electron microscopy (cryo-EM) structure of Myosin VI. For the first time, it revealed the structural basis of Myosin VI in the transition between its ‘On’ and ‘Off’ states.
Their work, entitled “Autoinhibition and activation of myosin VI revealed by its cryo-EM structure”, has been published in Nature Communications.

Figure 1. Cryo-EM structure of Myosin VI in its autoinhibited conformation
In this study, the research team successfully purified Myosin VI in complex with calmodulin (CaM) using in vitro recombinant expression systems. After multiple rounds of screening and optimization, they obtained near-atomic resolution cryo-EM maps of the Myosin VI-CaM complex and built the atomic structure (Figure 1). Structural analysis revealed that all domains of Myosin VI are involved in the formation of its autoinhibited conformation through complex interactions across the molecule. Importantly, several mutations associated with deafness were found to disrupt these interdomain interactions, likely impairing the regulatory activity of Myosin VI and leading to diseases.
During structural analysis, an intriguing phenomenon was observed. The single-alpha-helical extension region (SAH-E) at the tail end of Myosin VI was directly inserted into the head motor domain and blocked its ATPase activity. Thus, the SAH-E acted like a “brake” to inhibit the dynamics of this motor molecule, maintaining its closed state (Figure 2). Such a direct insertion into an active site to lock the head conformation was the first observation in the field of cytoskeletal motor proteins, significantly expanding our understanding of the regulatory mechanisms of myosins.

Figure 2. SAH-E acting like a ‘brake’ to be inserted into the motor head and inhibit its ATPase activity
Furthermore, through structural comparisons, it was found that in the autoinhibited conformation, all known cargo binding sites within the cargo-binding domains (CBD1 and CBD2) of Myosin VI were tightly shielded, effectively preventing cargo binding in the closed state. Through structural analysis and biochemical validation, the researchers confirmed that different cargos may play distinct roles in activating Myosin VI. For instance, cargo adaptors GIPC and CLCa may disrupt the autoinhibited conformation of Myosin VI by interfering with interdomain interfaces, while another cargo adaptor, Dab2, can mediate the formation of Myosin VI dimers, allowing it to sustain movement along actin filaments.
Based on these findings, the team proposed a working model for Myosin VI, illustrating its transition from autoinhibition to a cargo-activated state (Figure 3). In this model, Myosin VI autoinhibition was relieved upon cargo binding, followed by the adoption of monomeric or dimeric forms, thereby exerting its functions in transport or anchoring.
In conclusion, the study summarized the molecular basis of Myosin VI activity regulation and further deepened our understanding of cytoskeletal motors through comparisons with the autoinhibition structures of other myosins, such as Myosin V and Myosin II. This research breakthrough lays the foundation for the future development of therapeutic strategies for diseases associated with defects in intracellular material transport.
This work represents another significant advancement in the field of myosin research by Professor Wei, after previous contributions to the full-length structure of Myosin V in 2022 (paper link below), further elucidating the intricate regulatory mechanisms of molecular motors in intracellular transport.

Figure 3. Proposed model illustrating the ‘on-off’ mechanism of Myosin VI
Research Associate Professor Fengfeng Niu and graduate student Lingxuan Li are the co-first authors of the paper, with Associate Professors Zhiyi Wei and Cong Yu serving as corresponding authors. SUSTech is the first affiliation of the paper.
This research was supported by the National Natural Science Foundation of China (NSFC), Guangdong Basic and Applied Basic Research Fund, Shenzhen Municipal Science and Technology Innovation Committee, Shenzhen-Hong Kong Institute of Brain Science, Shenzhen Key Laboratory of Biomolecular Assembling and Regulation, and the SUSTech Cryo-EM Center and Core Research Facilities.
Paper links:
Nature Communications: https://www.nature.com/articles/s41467-024-45424-7
Previous related study in Science Advances: https://www.science.org/doi/epdf/10.1126/sciadv.add4187
To read all stories about SUSTech science, subscribe to the monthly SUSTech Newsletter.
Proofread ByAdrian Cremin, Yingying XIA
Photo BySchool of Life Sciences