With the development of industry, phosphonates, which are extensively used as scale inhibitors in reverse osmosis (RO) membrane treatment systems and cooling water circulation systems, accumulate in the effluent. Under the influence of light and microorganisms, phosphonates continuously release phosphate ion (PO43−), exacerbating eutrophication in water bodies and leading to pollution such as algal blooms. Due to the strong, complex capacity of phosphonates on metal ions and their highly stable molecular structure, existing wastewater treatment technologies have poor treatment performance, often requiring extended treatment times or the consumption of a large amount of energy.
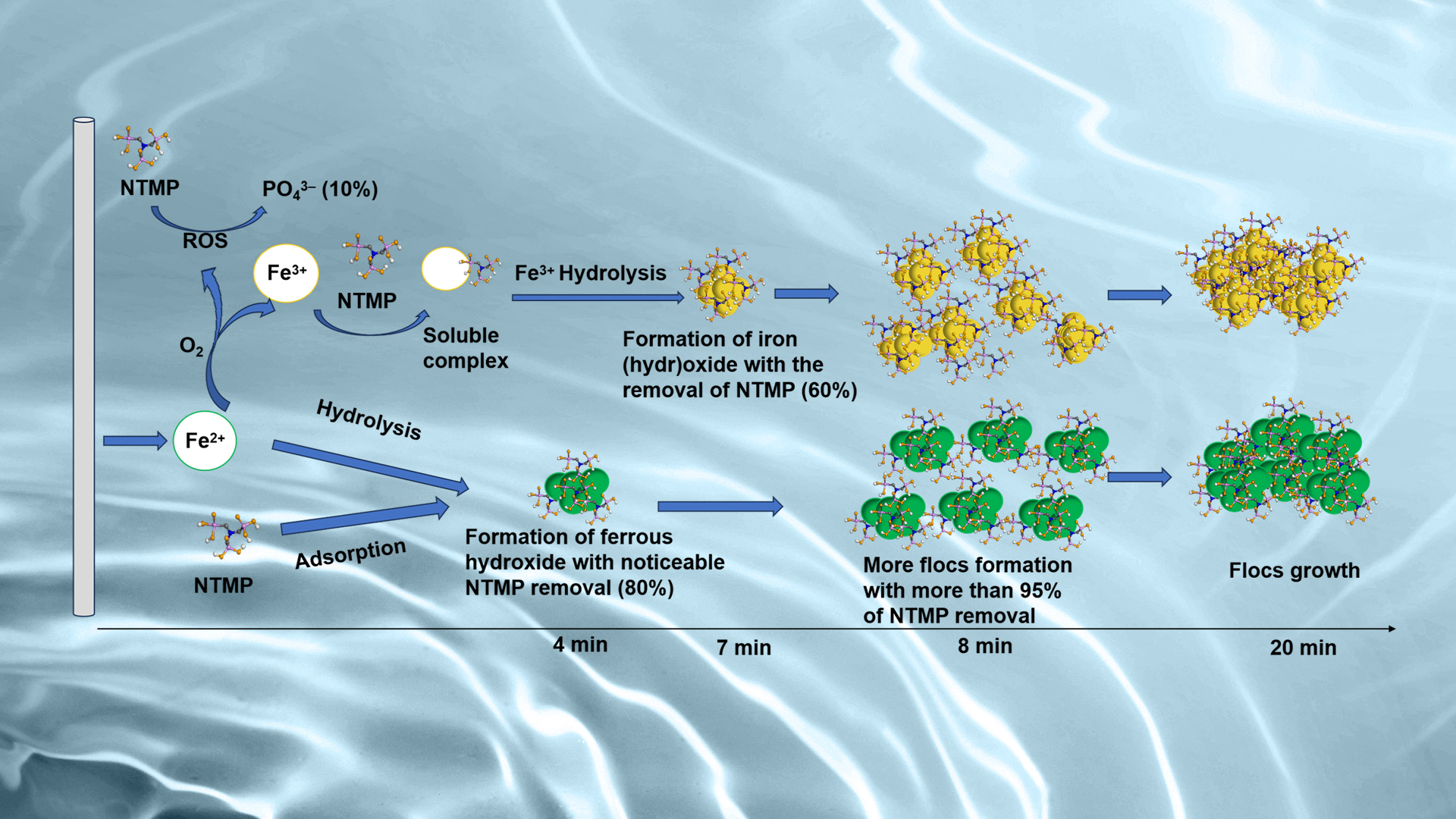
In response to this issue, the research group led by Assistant Professor Yang Lei from the School of Environmental Science and Engineering at the Southern University of Science and Technology (SUSTech) has recently published a study that reveals the significant role of iron ion complexation and flocculation processes in the Fe-electrocoagulation (Fe-EC) system for the removal of phosphonates from water. For the first time, it was found that under specific anoxic conditions, the removal efficiency of phosphonates by the Fe-EC system was significantly higher than under oxic conditions.
Their findings, entitled “Importance of iron complexation and floc formation towards phosphonate removal with Fe-Electrocoagulation”, have been published in the international journal Water Research. The researchers studied the removal performance of phosphonates by the Fe-EC system, which is cost-effective, controllable in conditions, and capable of in-situ flocculant production under various conditions. They also conducted an in-depth discussion on the mechanism of phosphonate removal by the Fe-EC system.
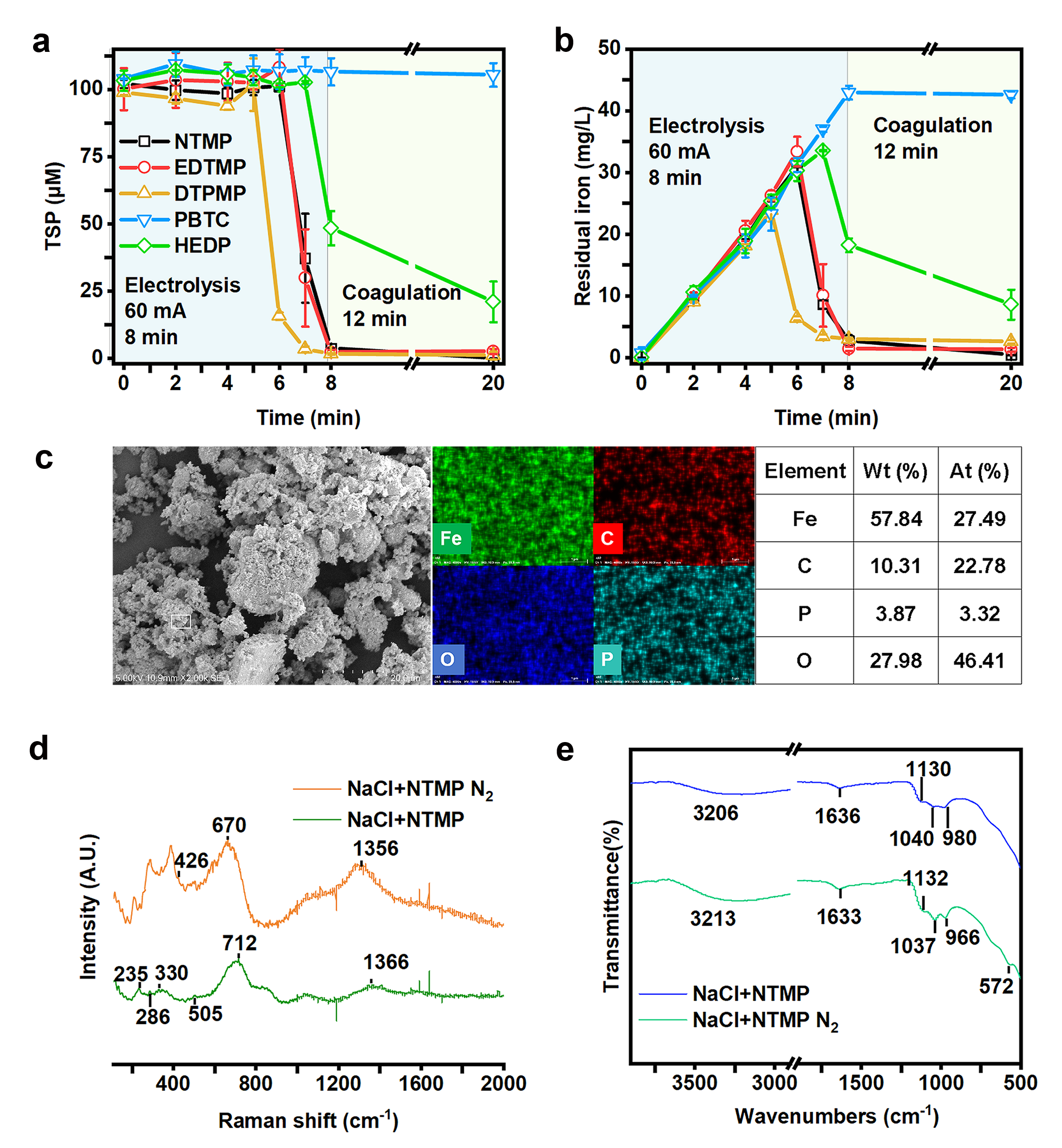
Figure 1. The change of TSP (Total soluble phosphorus) (a) and residual iron (b) concentration in removing 5 types of phosphonates by Fe–EC in the presence of O2. The SEM-EDS image (c) of solid generated from NTMP experiments under oxic conditions. The Raman (d) and FTIR (e) spectrums of products in NTMP experiments under oxic and anoxic conditions.
They selected five common phosphonate scale inhibitors as experimental subjects to verify the removal performance of phosphonates by the Fe-EC system under different conditions. During the experimental process, it was found that phosphonates were adsorbed by generated iron (hydr)oxides by the formation of Fe-O-P bonds (Figure 1). Under oxic conditions, a small number of phosphonates were also degraded by reactive oxidizing species generated during the oxidation process of ferrous ions. However, the growth of the flocs also consumed some of the binding sites with phosphonates, leading to decreased removal performance.
Surprisingly, the experimental results indicated that under anoxic conditions (Figure 2), the removal performance of phosphonates by Fe-EC was significantly higher than under oxic conditions (Figure 1), achieving higher removal efficiency with less iron and energy consumption. This demonstrated that the complexation of (ferrous) iron ions played a positive role in the Fe-EC system’s removal of phosphonates. Anoxic or oxygen-deficient environments are more common in real wastewater treatment systems. This finding is conducive to the development of anoxic Fe-EC reactors to achieve more efficient removal of phosphonates.
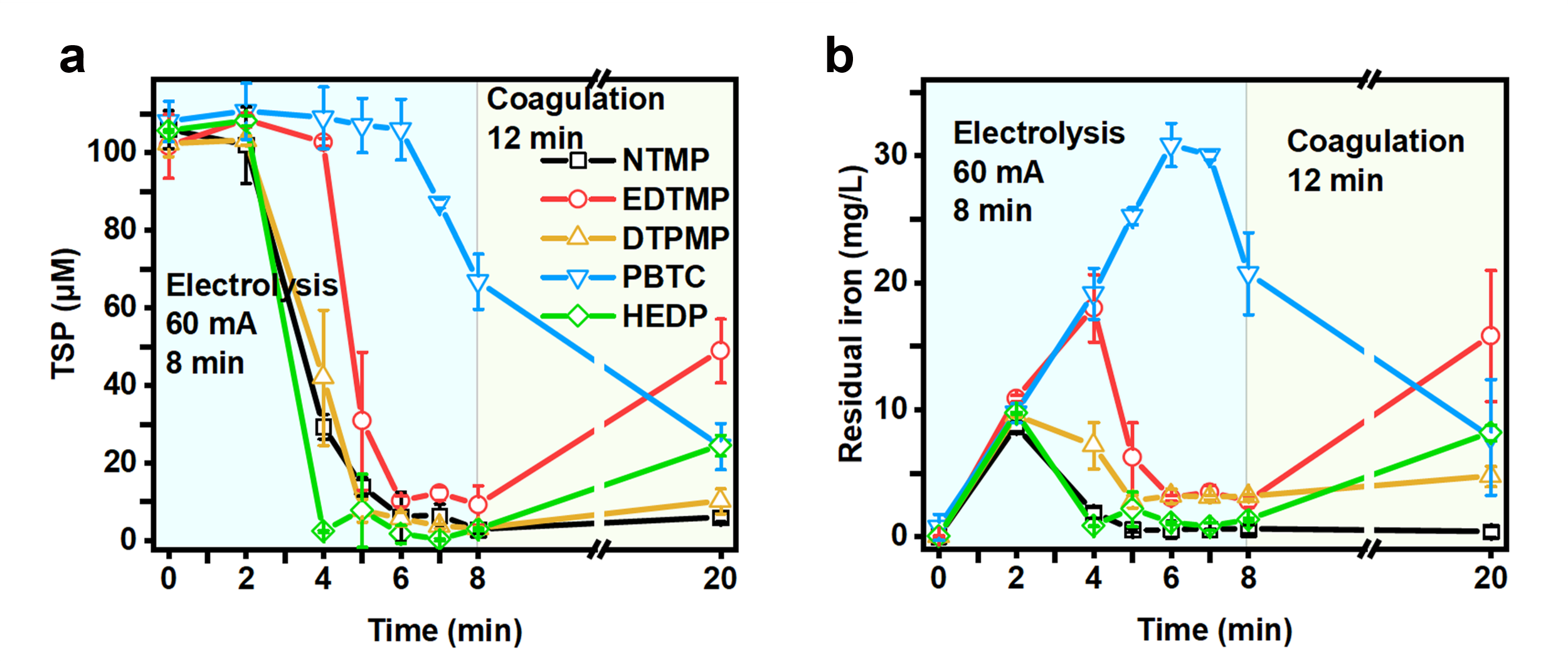
Figure 2. The change of TSP (a) and residual iron (b) concentration in removing different phosphonates under anoxic conditions in the Fe-EC system.
Apart from the mechanism study, the researchers further tested the performance of the Fe-EC system in removing phosphonates from complex water environments and assessed its economic value. The results showed that the coexisting substances in actual cooling water (such as Ca2+, etc.) had a bridging and strong neutralization effect that promoted the removal of phosphonates. Under oxic conditions, TSP concentration decreased to below 0.5 mg/L (Figure 3) after only 2 min of electrolysis, meeting the discharge standards. According to calculations, the operational expenditure for treating one ton of actual cooling water was only € 0.009, which was lower than the costs associated with advanced oxidation and chemical coagulation processes.
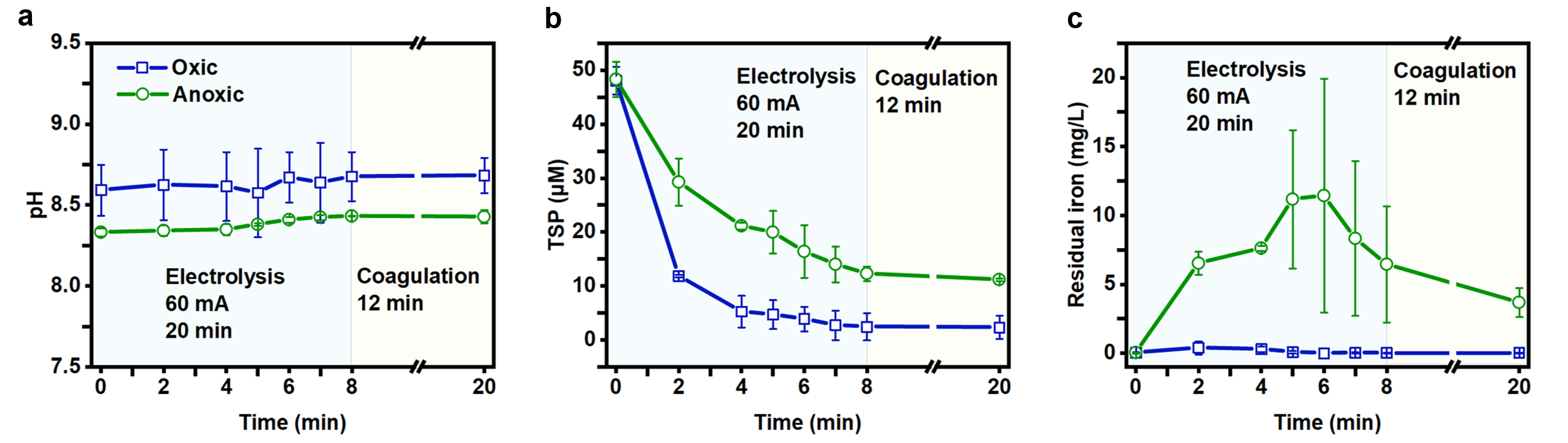 Figure 3. The change of pH value (a), TSP (b) and residual iron (c) concentration in the treatment of actual cooling water.
Figure 3. The change of pH value (a), TSP (b) and residual iron (c) concentration in the treatment of actual cooling water.
This study holds significant fundamental theoretical importance and potential application value. It clarifies the importance of iron ion complexation and floc formation in the Fe-EC system for the removal of phosphonates. It was discovered that the Fe-EC system’s removal efficiency for phosphonates under anoxic conditions was significantly higher than under oxic conditions. This finding is conducive to the development of novel Fe-EC reactors in real wastewater treatment processes that are anoxic or oxygen-deficient, bringing new insights for the development of novel phosphonates removal processes.
Haiyang Hu, a master’s student at SUSTech, is the first author of the paper. Postdoctoral fellow Bingnan Song is a co-author, and Yang Lei is the corresponding author. SUSTech is the sole affiliated institution.
The research was supported by the Guangdong Basic and Applied Basic Research Foundation, Shenzhen Basic Research Program (Shenzhen Natural Science Foundation), Shenzhen Higher Education Institutions Stability Support Program, Shenzhen Key Laboratory of Precision Measurement and Early Warning Technology for Urban Environmental Health Risks, High-Level of Special Fund, and the SUSTech Start-Up Fund.
Paper link: https://doi.org/10.1016/j.watres.2024.122117
To read all stories about SUSTech science, subscribe to the monthly SUSTech Newsletter.
Proofread ByAdrian Cremin, Yingying XIA
Photo BySchool of Environmental Science and Engineering