Electrochemical devices rely on the adsorption, desorption, and redox reactions at the solid-liquid and solid-solid interfaces of functional material layers to achieve energy storage, molecular-level physiological detection, and biomimetic functions. Consequently, the micro-nano interface evolution mechanisms and component optimization of device functional materials significantly influence their electrochemical performance. As the demand for highly stable and efficient electrochemical systems grows, understanding these complex interactions is essential to improving device stability and performance, paving the way for advancements in sustainable energy and health technologies.
To address the aforementioned issue, the research group of Assistant Professor Yuanjing Lin from the Southern University of Science and Technology (SUSTech) has made new progress in the design of highly stable electrochemical materials with an in-depth study of device performance degradation mechanisms. Together with collaborators, they conducted studies on material components and interface structure regulation strategies for multifunctional micro-nano electrochemical devices. They have published four research papers in academic journals, including Nature Nanotechnology, Nature Communications, Energy & Environmental Science, and Advanced Materials.
Addressing degradation in lithium-ion cathodes
To study the stability of electrochemical devices, such as performance degradation during continuous redox reactions, the group collaborated with multiple teams to analyze the structural evolution of nanomaterial particles and reaction interfaces, revealing the mechanisms of performance degradation. Their study found that lithium-rich manganese-based oxides underwent phase transitions from layered to spinel structures on the particle surfaces during cycling, providing conditions for photocatalytic reactions and thermal reconstruction (Figure 1).
Based on this, the researchers innovatively proposed a strategy to enhance the electrochemical stability of materials using photothermal radiation. This work provides a novel strategy for restoring electrochemical material performance, mitigating structural degradation, and constructing high-performance electrochemical devices.
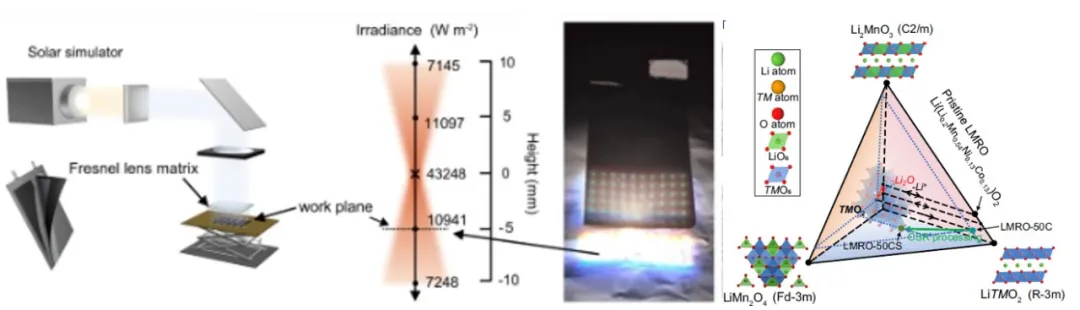
Figure 1. Schematic of the chemical composition and lattice structure evolution of lithium-rich manganese-based oxides before and after electrochemical cycling and photothermal radiation treatment
The related findings were published under the title “Revealing the degradation pathways of layered Li-rich oxide cathodes” in Nature Nanotechnology.
Visiting student Hailong Wang from SUSTech is the co-first author of this paper. Assistant Professor Yuanjing Lin, Professor Xin He from Sichuan University, Professor Jie Li from the Polytechnic University of Milan, and Professor Jun Lu from Zhejiang University served as co-corresponding authors.
Another paper titled “Efficient direct repairing of lithium- and manganese-rich cathodes by concentrated solar radiation” was published in Nature Communications.
Hailong Wang is the first author of the paper, with Assistant Professor Yuanjing Lin, Professor Xin He, and Professor Jun Lu serving as co-corresponding authors.
Advancing zinc-ion battery stability and safety
Based on a theoretical study, the group investigated construction strategies for high-performance aqueous zinc-ion batteries. Aqueous zinc-ion storage devices feature high safety and environmental friendliness. However, they still face multiple challenges in practical applications, such as electrode reversibility and cycling life, making it difficult to achieve simultaneous optimization of the anode and cathode.
They introduced imidazolium bromide (MPIBr) into the electrolyte, designing and preparing a fully ionic-utilized aqueous electrolyte that enables dendrite-free and shuttle effect-free Zn-Br2 batteries (Figure 2). For the Zn anode, the MPI+ cation effectively suppresses water-related side reactions and promotes uniform Zn deposition, while the Br− anion participates in the solvation structure of Zn2+, facilitating rapid migration and desolvation of Zn2+. For the Br2 cathode, the MPI+ cation exhibited strong chelation with polybromide ions, effectively inhibiting the shuttle effect of polybromides, thereby enhancing the battery’s stability. Furthermore, the Br− anions and Zn2+ cations in the electrolyte can construct a dual-electrode Zn-Br2 battery in situ during charging, avoiding the complex steps involved in preparing active materials for both electrodes. The battery can stably cycle 1000 times at a 100% discharge depth.
This work profoundly analyzes the impact mechanisms of electrolyte composition on the electrode-electrolyte interface, aiding the development of high-performance, low-cost zinc-halogen batteries.
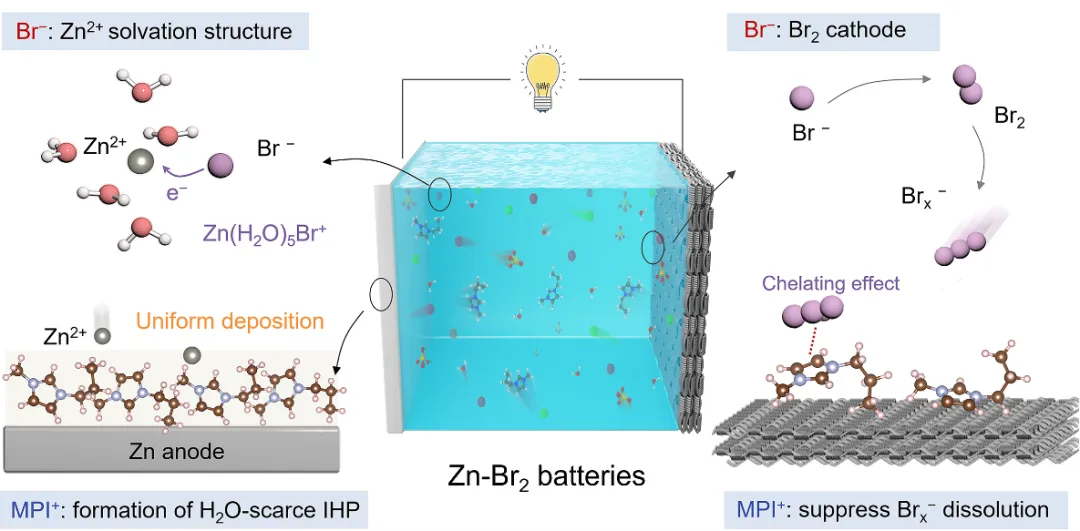
Figure 2. Aqueous zinc-bromine battery with fully utilized imidazolium bromide electrolyte achieving high stability
These findings were published under the title “Fully exploited imidazolium bromide for simultaneous resolution of cathode and anode challenges in zinc–bromine batteries” in Energy & Environmental Science.
Postdoctoral Researcher Linyu Hu from SUSTech is the first author of this paper. Assistant Professor Yuanjing Lin and Professor Xin He are the co-corresponding authors, with SUSTech serving as the first affiliated institution.
Sweat-activated power solutions for wearables
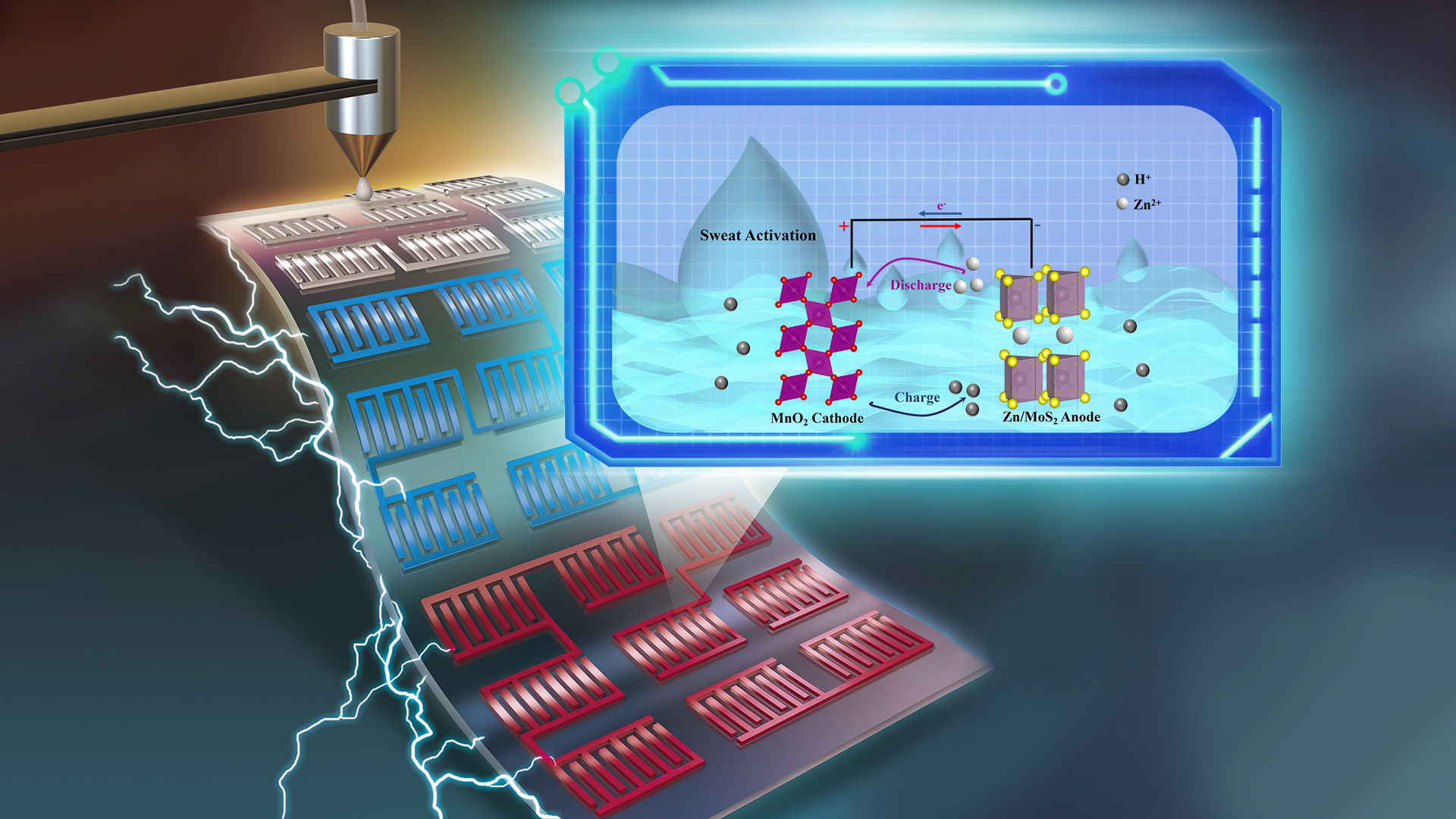
In addition, the research group developed a fully printed, sweat-activated micro zinc/molybdenum disulfide (Zn/MoS2)-manganese dioxide (MnO2) battery with long-term stability and high capacity, which can be integrated into flexible wearable devices (Figure 3). The battery anode employs two-dimensional MoS2 to achieve lattice matching with Zn powder, enhancing the stability of the zinc anode and promoting the efficiency of electron and ion transport.
By utilizing mildly acidic sweat, it eliminates by-products on the MnO2 cathode and compensates for water loss in the hydrogel electrolyte, activating performance and extending battery life. The battery is fabricated using layered printing technology to achieve patterned designs. At a current density of 0.16 mA cm-2, it can achieve a specific capacity of 318.9 μAh cm-2 and an energy density of 424.6 μWh cm-2, maintaining approximately 90% cycling stability after 250 cycles. This sweat-activated micro-battery offers a feasible strategy for environmentally friendly energy storage technology in wearable devices.
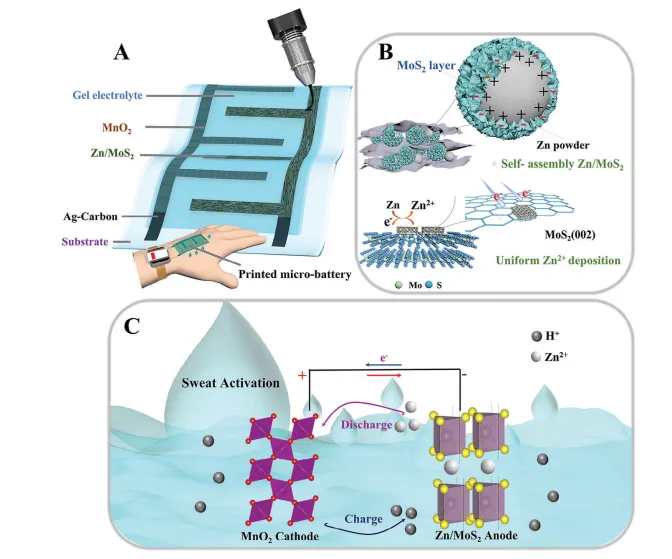
Figure 3. Fully printed and sweat-activated micro-battery based on molybdenum disulfide-modified zinc anode
These findings were published under the title “Fully Printed and Sweat-Activated Micro-Batteries with Lattice-Match Zn/MoS2 Anode for Long-Duration Wearables” in Advanced Materials.
Ph.D. student Xinyi Zhang from SUSTech is the first author of this paper. Assistant Professor Yuanjing Lin is the corresponding author, with SUSTech serving as the first affiliated institution.
Paper links (In order of appearance above):
Nature Nanotechnology: https://www.nature.com/articles/s41565-024-01773-4
Nature Communications: https://www.nature.com/articles/s41467-024-45754-6
Energy & Environmental Science: https://pubs.rsc.org/en/Content/ArticleLanding/2024/EE/D4EE02096K
Advanced Materials: https://onlinelibrary.wiley.com/doi/10.1002/adma.202412844
To read all stories about SUSTech science, subscribe to the monthly SUSTech Newsletter.
Proofread ByAdrian Cremin, Yingying XIA
Photo By