Opioid drugs are the most commonly used analgesics for treating moderate to severe pain in clinics. While playing a major role in opioid analgesics, the μ-opioid receptor (MOR) can lead to unwanted side effects, such as respiratory inhibition, addiction, and opioid-induced constipation, due to its broad expression in the nervous system. Pruritus, or itch, is another common side effect of opioid analgesics.
Clinical research has reported that pruritus occurs in 30%-100% of cases following epidural or intrathecal administration of opioid analgesics. Conversely, MOR antagonists, like naloxone, naltrexone, and nalbuphine, have proven effective in mitigating chronic itch associated with dermatitis, cholestatic liver disease, uremic disease, and programmed cell death protein 1(PD1) immunotherapy. Despite numerous studies on the mechanism of itch sensation generation in both peripheral and central nervous systems, the nature of MOR signaling in itch transmission remains poorly understood.
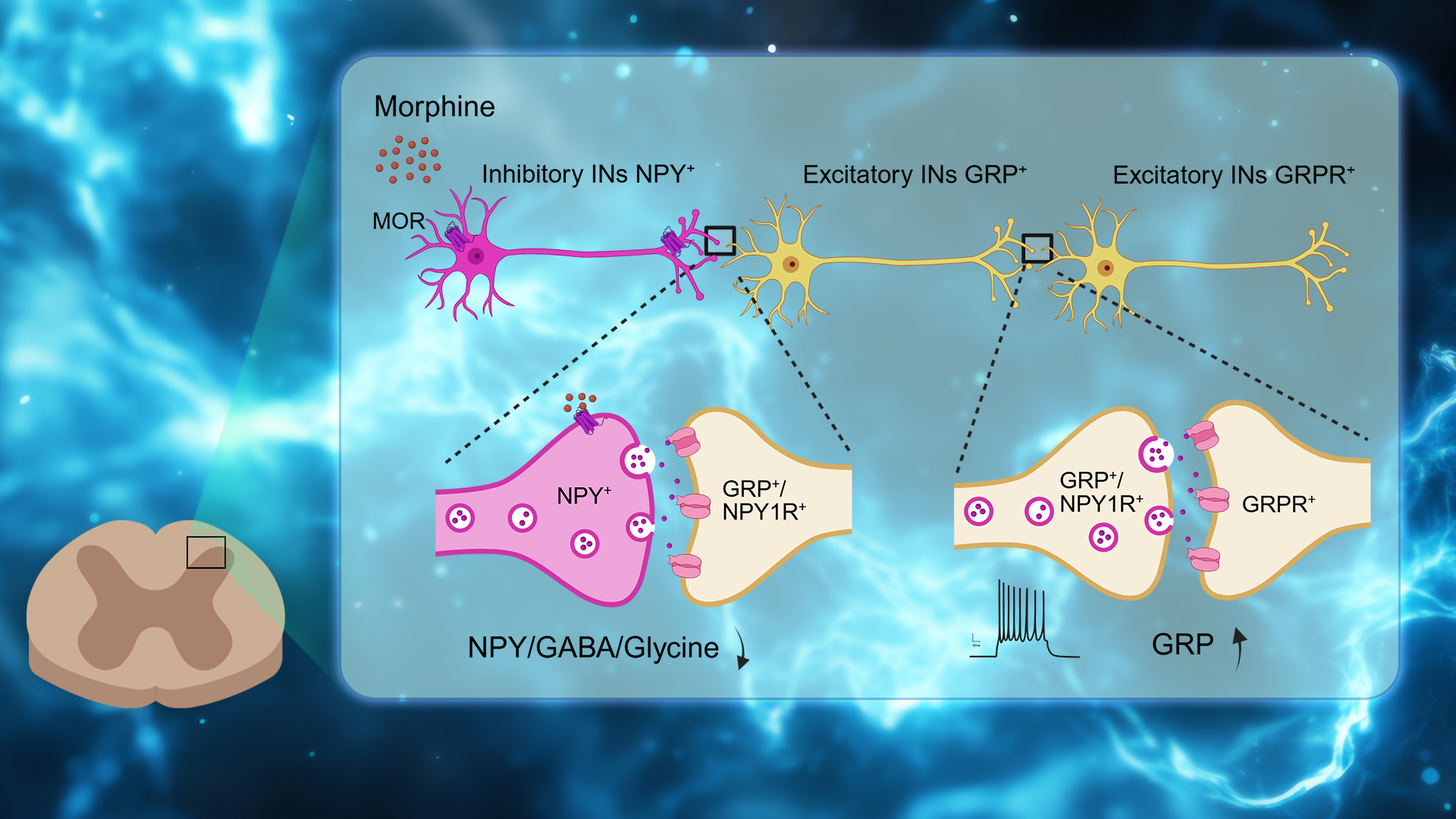
Assistant Professor Zilong Wang’s research team from the School of Medicine at the Southern University of Science and Technology (SUSTech), in collaboration with Professor Chaoran Wu from the First Affiliated Hospital of SUSTech and Associate Researcher Changyu Jiang from the Department of Pain Medicine at the Sixth Affiliated Hospital of Shenzhen University Medical School, has systematically revealed the cellular, molecular, and neural circuit mechanisms underlying the pruritus side effect caused by commonly used opioid analgesics. Their study found that the activation of MOR expressed by Neuropeptide Y (NPY)+ inhibitory neurons mediated the pruritus induced by opioids by “disinhibiting” the downstream GRP-GRPR microcircuit.
Their work, titled “Neuropeptide Y neurons mediate opioid-induced itch by disinhibiting GRP-GRPR microcircuits in the spinal cord,” has been published in the journal Nature Communications.
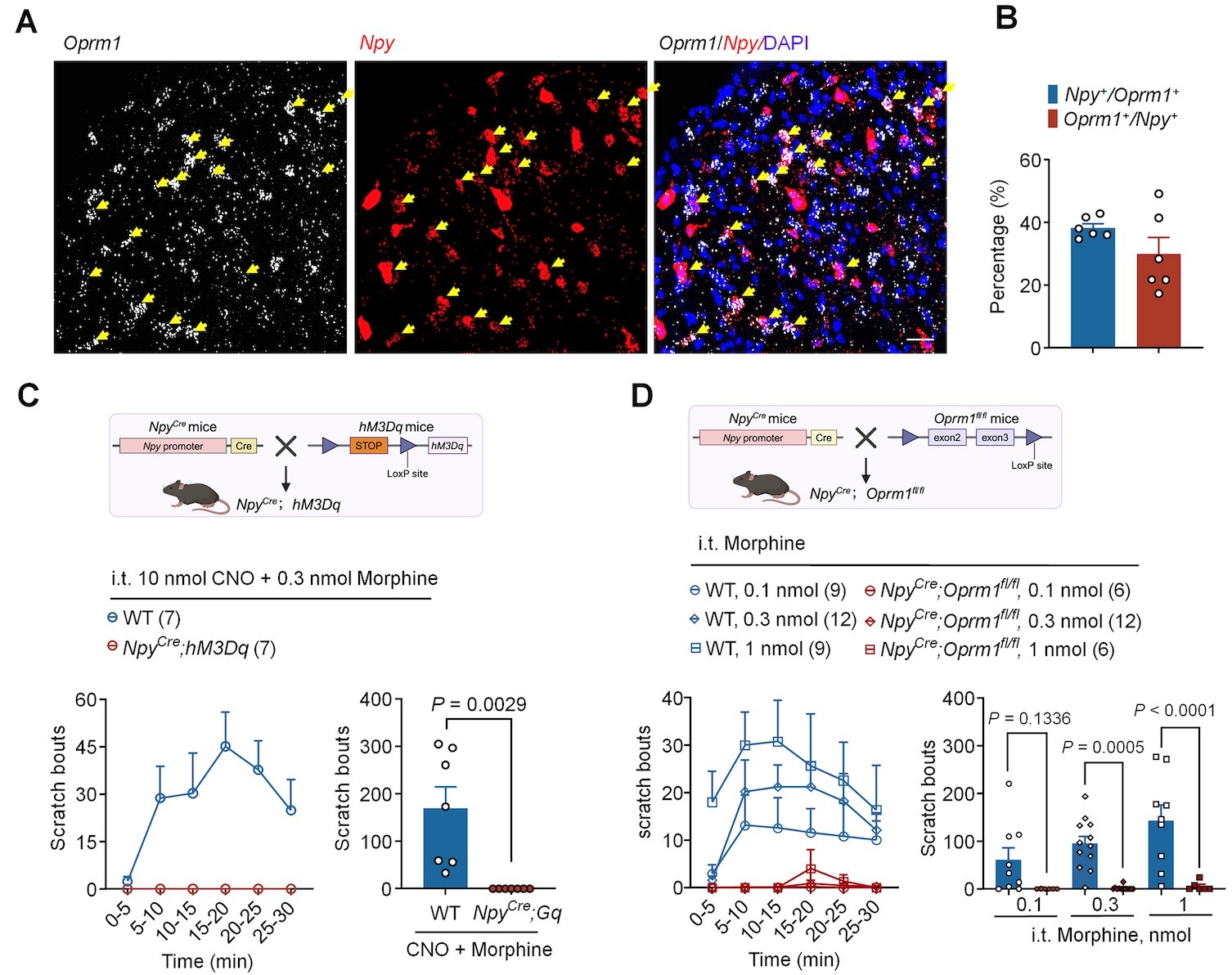
Figure 1. Opioid-induced itch is mediated by MOR on the NPY+ interneurons
The researchers report the role of NPY+ neurons and their downstream circuits in mediating opioid-induced pruritus. In the dorsal horn of the spinal cord, MOR is highly expressed in NPY-positive neurons, which has an important regulatory function on the pruritus neural circuit. Conditional knockout of MOR in NPY neurons (NpyCre; Oprm1fl/fl) completely abolishes the pruritus response caused by opioids. It is the first report that discovered that the MOR expressed on NPY+ inhibitory neurons mediating the pruritus induced by opioids.
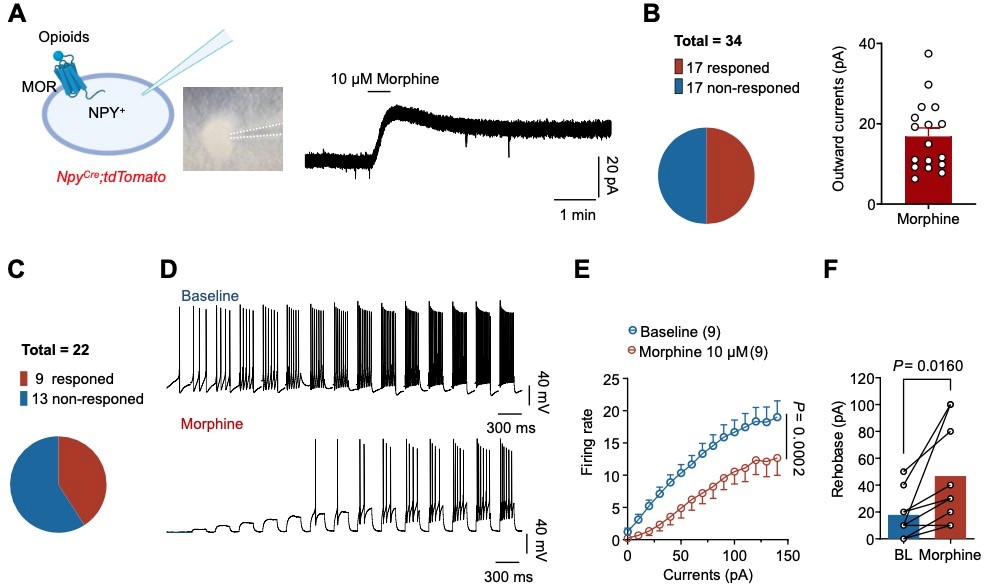
Figure 1. Opioid-induced itch is mediated by MOR on the NPY+ interneurons
The researchers report the role of NPY+ neurons and their downstream circuits in mediating opioid-induced pruritus. In the dorsal horn of the spinal cord, MOR is highly expressed in NPY-positive neurons, which has an important regulatory function on the pruritus neural circuit. Conditional knockout of MOR in NPY neurons (NpyCre; Oprm1fl/fl) completely abolishes the pruritus response caused by opioids. It is the first report that discovered that the MOR expressed on NPY+ inhibitory neurons mediating the pruritus induced by opioids.
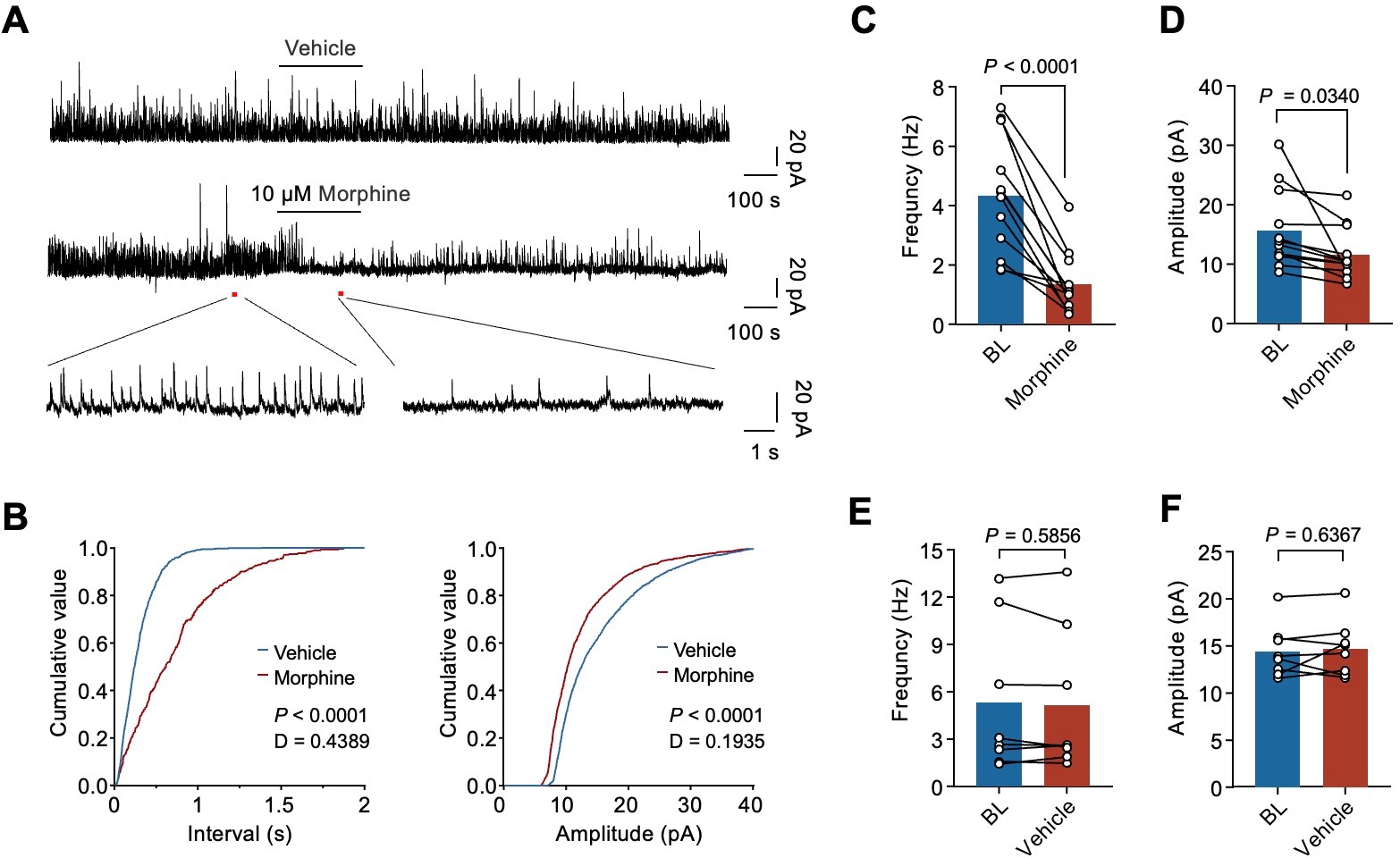
Figure 3. Morphine impairs inhibitory synaptic transmission in GRP+ interneurons in the SDH
Gastrin releasing peptide (GRP) and its receptor GRPR play important roles in the pruritus pathway. The anterograde and retrograde virus tracer indicated that NPY+ interneurons provide monosynaptic inputs to GRP+ interneurons. The RNAscope data showed that Npy1r and Grp mRNA expressions have remarkably high co-localization (66%) in the lamina II of SDH.
This work uncovered the critical roles of the GRP-GRPR pathway in opioid-induced itch. Morphine-induced itch was dramatically decreased in the GRP+ neurons-ablated mice (GrpCre;iDTR) or by chemogenetic inhibition of GRP+ interneurons in GrpCre;hM4Di mice. Furthermore, morphine-induced itch was decreased in GRP conditional knockout mice (Vglut2Cre;Grpfl/fl), directly showing that GRP itself is essential for morphine-induced itch. Lastly, GRPR antagonist PD176252 significantly inhibited morphine-induced itch, which is consistent with previous results from GRPR knockout mice.
These findings demonstrate that intrathecal morphine elicits itch via acting on MOR on NPY+ neurons in SDH, leading to the disinhibition of GRP-GRPR microcircuits to elicit itch.
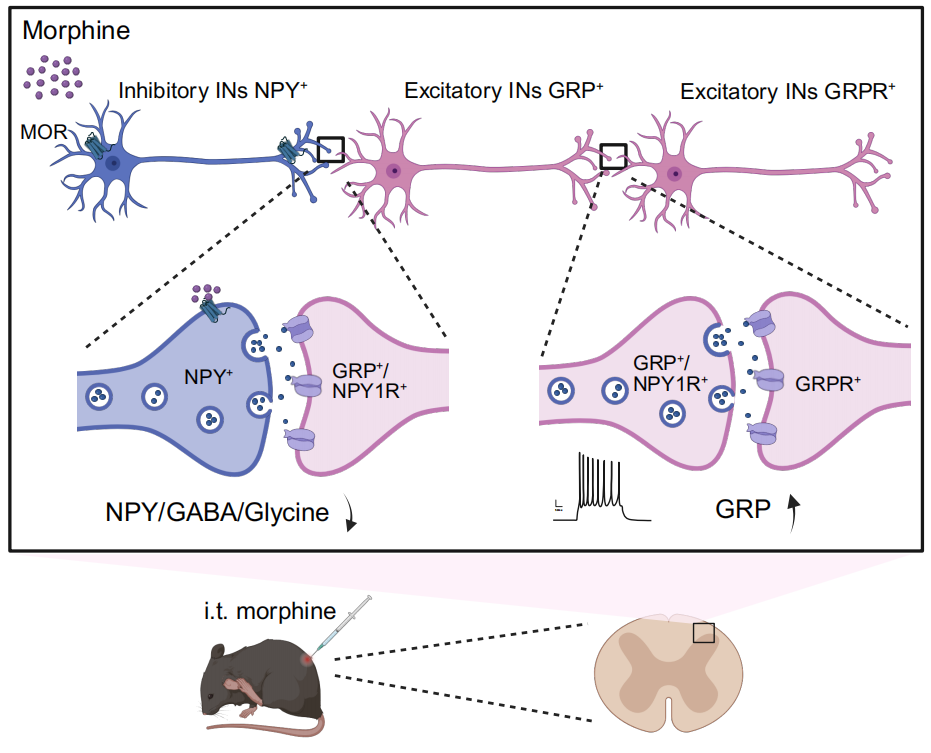
Figure 4. A schematic diagram illustrates the morphine-induced itch in the spinal circuit; Morphine acts on MORs expressed on NPY+ inhibitory interneurons and causes disinhibition of GRP+/NPY1R+ excitatory interneuron, then further activates GRPR+ excitatory interneuron to elicit itch
Doctoral students Qian Zeng and Yifei Wu, together with master’s student Yitong Li, are the co-first authors of the paper. Assistant Professor Zilong Wang, Professor Chaoran Wu, and Associate Researcher Changyu Jiang are the co-corresponding authors, and SUSTech is the first affiliated institution.
Paper link: https://www.nature.com/articles/s41467-025-62382-w
To read all stories about SUSTech science, subscribe to the monthly SUSTech Newsletter.
Proofread ByAdrian Cremin, Yifei REN
Photo BySchool of Medicine